Vaccine abundance: Israel
In mid-March, 50% of the population was fully vaccinated and an additional 10% had received the first vaccine dose (Figure 1). Of note, with more than 2 million children who cannot yet be vaccinated, it will be instructive to see how the number of cases will evolve after lifting of most lockdown restrictions on 7 March.
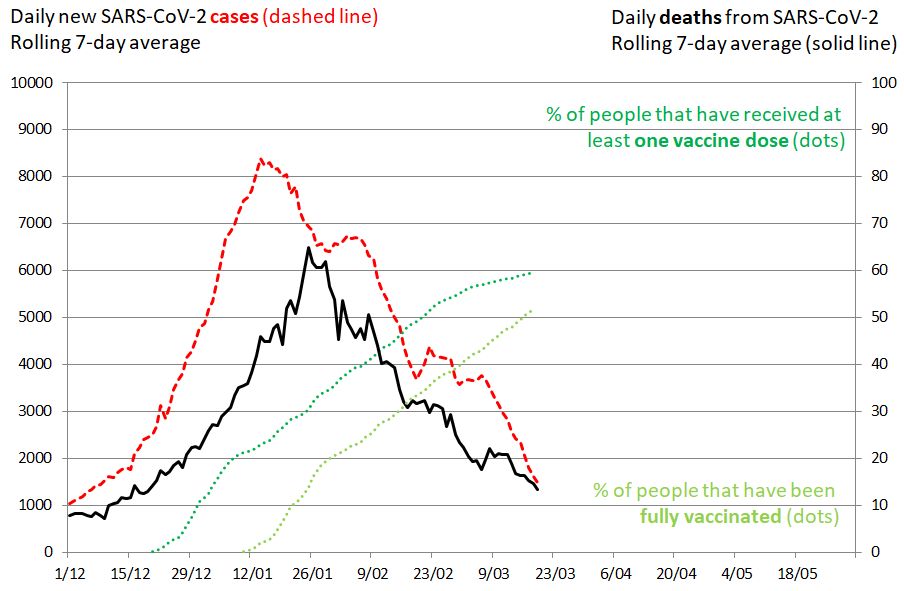
Figure 1. SARS-CoV-2 cases in Israel. 19 March 2021. Impact of mass vaccination on the pandemic. The rolling 7-day average of new SARS-CoV-2 cases is shown in red (left vertical axis), the rolling 7-day average of deaths as a plain black line (right vertical axis). The percentage of people that have received at least one vaccine dose is shown in green. The percentage of people that have been fully vaccinated is shown in green-yellow. The evolution of daily new cases and deaths were influenced by lockdown measures, transmissibility of circulating viruses and the vaccination campaign.
Vaccine scarcity: Continental Europe
The English B.1.1.7 variant has reached Continental Europe (Figure 2). A third of France’s population and most of Italy’s is again living under lockdown, and other countries like Germany will soon follow. The sputtering vaccination campaign will have no significant impact on the B.1.1.7 epidemic over the next two months. Until the beginning of the summer season, Europe will have to rely on NPI’s (non-pharmaceutical interventions) to mitigate the impact of B.1.1.7.
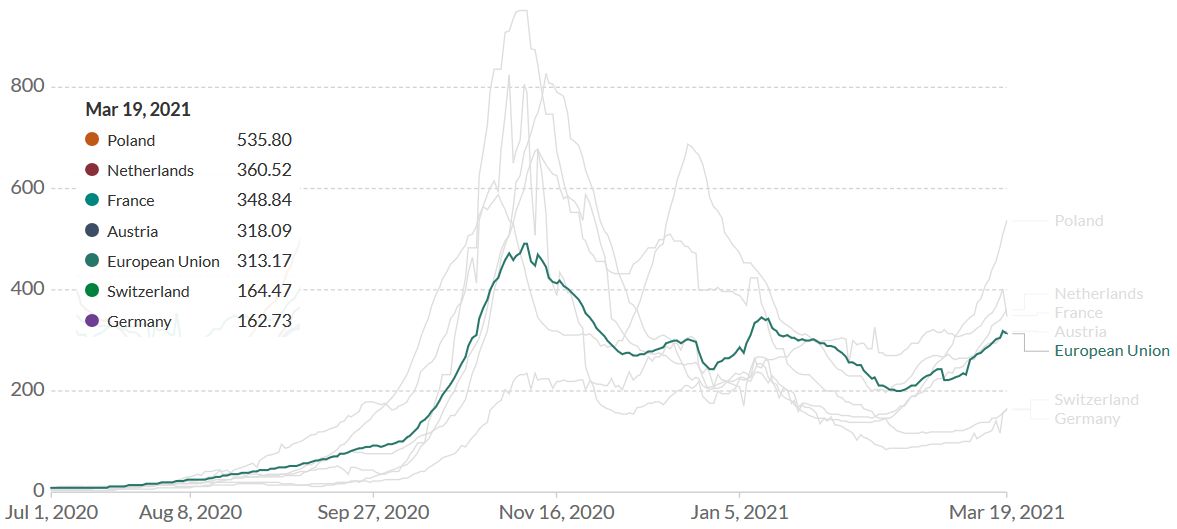
Figure 2. The English B.1.1.7 variant has reached Continental Europe. Shown is the rolling 7-day average per million people for selected European countries. To recalculate these values as the ‘cumulative 7-day rolling incidence per 100.000 people’, multiply the values by 0.7. Example: the March 19 value for the European Union, 313, is equivalent to a cumulative 7-day incidence of 219/100.000 people. Source and copyright: Our World in Data – Johns Hopkins University CSSE COVID-19 Data, accessed 20 March.
Press reports
- The Pfizer-BioNTech vaccine may have 97% efficacy against disease and death and 94% against infection without symptoms (press release). | Boseley S. Israeli real-world data on Pfizer vaccine shows high Covid protection. The Guardian 2021, published 11 March. Full-text: https://www.theguardian.com/world/2021/mar/11/israeli-real-world-data-on-pfizer-vaccine-shows-high-covid-protection
- These (press release) data will help you interpret other (press release) data by Novavax (Novavax 20210311) that announce ‘100% protection against severe disease, including all hospitalization and death’. Such claims should not be taken at face value until ‘Israeli-style’ country-wide data are available. Novavax also announced that efficacy of their vaccine candidate in a Phase IIb trial taking place in South Africa (a region where the vast majority of strains were B.1.351) was only 55.4% among the HIV-negative trial participants. It would have been interesting to have details on the efficacy among HIV-positive participants. 30%? 20%? Even less? | Novavax 20210311. Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. Novavax 2021, 11 March. Link: https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0
Important publications of the past two weeks
Good B.1.1.7 news
- Using a lentivirus-based pseudovirus assay, Xiaoying Shen and colleagues show that B.1.1.7 is unlikely to be a major concern for current vaccines or for an increased risk of reinfection. | Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral Spike vaccines. Cell Host Microbe March 03, 2021. https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(21)00102-5
- Again data indicating that the B.1.1.7 lineage will not escape BNT162b2-mediated protection. | Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021 Mar 12;371(6534):1152-1153. PubMed: https://pubmed.gov/33514629. Full-text: https://doi.org/10.1126/science.abg6105 | See also the comment by Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science. 2021 Mar 12;371(6534):1103-1104. PubMed: https://pubmed.gov/33707254. Full-text: https://doi.org/10.1126/science.abg7404
Bad B.1.1.7 news (er, continue with NPIs)
- Vaccination alone might not be sufficient to contain the pandemic (Moore 2021; see also the comment by Contreras & Priesemann 2021). In a modelling study, Moore et al. assumed default vaccine uptake of 95% in those aged 80 years and older, 85% in those aged 50–79 years, and 75% in those aged 18–49 years. Even with the optimistic assumption that the vaccine will prevent 85% of infections, the authors estimate R to be 1.58 (95% credible intervals [CI] 1.36–1.84) once all eligible adults have been offered both doses of the vaccine. | Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis 2021, published 18 March. Full-text: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00143-2/fulltext | See also the comment by Contreras S, Priesemann V. Risking further COVID-19 waves despite vaccination. Lancet Infect Dis 2021, published 18 March. Full-text: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00167-5/fulltext
Good and bad B.1.351 news
- B.1.351 and P.1 were able to infect common laboratory mice, replicating to high titers in the lungs. The mutations of these variants may therefore not only enhance transmission and antibody evasion but also expand host range (bad news) and provide a new experimental model (good news). | Montagutelli X, Prot M, Levillayer L, et al. The B.1.351 and P.1 variants extend SARS-CoV-2 host range to mice. bioRxiv 2021, posted 18 March. Full-text: https://www.biorxiv.org/content/10.1101/2021.03.18.436013v1
Immune escape
- Protection conferred by the mRNA-1273 vaccine against the P.1, B.1.427/B.1.429, and B.1.351 variants remains to be determined. | Wu K, Werner AP, Koch M. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. NEJM March 17, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2102179
- In The great escape?, Jérémie Prévost and Andrés Finzi (Prévost & Finzi 2021) describe three articles about new mutations in the SARS-CoV-2 spike receptor binding domain that escape neutralizing responses. The articles highlight ‘the importance of surveillance of SARS-CoV-2 evolution to anticipate and manage new variants that could impact reinfection, vaccine efficacy, and immunotherapies’:
- Liu Z et al. https://doi.org/10.1016/j.chom.2021.01.014 – Hard time ahead for monoclonals.
- Thomson et al. https://doi.org/10.1016/j.cell.2021.01.037 – Serum antibody binding is predominantly affected by mutations at just a few dominant epitopes in the RBD. The most important site is E484, where neutralization by some sera is reduced > 10-fold by several mutations.
- Greaney et al. https://doi.org/10.1016/j.chom.2021.02.003 – the N439K mutation confers resistance against several neutralizing monoclonal antibodies, including one authorized for emergency use by the FDA, and reduces the activity of some polyclonal sera from persons recovered from infection.
Clinical consequences
- 184,786 B.1.1.7 positive people from a research platform that covers 40% of England’s population registered with a general practitioner. After controlling for comorbidities, age, week, region & other sociodemographics, the authors found a 67% increased risk of death for B.1.1.7 compared with non-B.1.1.7 cases. | Grint DJ, Wing K, Williamson E, et al. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Volume 26, Issue 11, 18 March 2021. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.11.2100256
- An analysis of 54,906 matched pairs of people (who tested positive for SARS-CoV-2 between 1 October 2020 and 29 January 2021, followed-up until 12 February 2021) found a mortality hazard ratio of 1.64 associated with B.1.1.7 infection in the community. | Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9;372:n579. PubMed: https://pubmed.gov/33687922. Full-text: https://doi.org/10.1136/bmj.n579
Eeek!
- The variant circulating in South Africa (B.1.351) and the P1 isolate from Brazil have both acquired E484K – a change at position 484 from glutamic acid (E) to lysine (K). It’s a change from acid to alkali, from a highly negative charge to a highly positive charge. This one mutation does seem to impair antibody binding, and is making vaccines less effective. In one study in South Africa, the Oxford-AstraZeneca vaccine didn’t show a convincingly protective effect against the B.1.351 variant. | Beale R. Eeek! [E484K] LRB 2021, published 4 March. Full-text: https://www.lrb.co.uk/the-paper/v43/n05/rupert-beale/eeek
Peer-reviewed former preprints
- The Wibmer paper shows that B.1.351 completely escapes three classes of therapeutically relevant antibodies. | Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nature Medicine 02 March 2021. https://www.nature.com/articles/s41591-021-01285-x
- The brilliant Wang paper. While B.1.1.7 is refractory to neutralization by many monoclonal antibodies, but not more resistant to convalescent plasma (CP) or vaccinee sera (VS), B.1.351 is not only refractory to neutralization by almost all mAbs but also by CP (9.4 fold) and VS (10.3-12.4 fold). | Wang P, Nair MS, Liu L, et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature March 8, 2021. https://www.nature.com/articles/s41586-021-03398-2
- The not less brilliant Tegally paper about the detection of B.1.351. | Tegally H, Wilkinson E, Giovanetti M, et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature. 2021 Mar 9. PubMed: https://pubmed.gov/33690265. Full-text: https://doi.org/10.1038/s41586-021-03402-9
- The worrying Collier paper. Sera from people vaccinated with the Pfizer-BioNTech vaccine exhibited a broad range of neutralizing titers against the historical virus and only modestly reduced against B.1.1.7 variant. But… add the E484K mutation, and everything changes. The authors conclude that an E484K emergence on a B.1.1.7 background represents a threat to the Pfizer-BioNTech vaccine. | Collier DA, De Marco A, Ferreira IA, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature March 11, 2021. https://www.nature.com/articles/s41586-021-03412-7
- The AstraZeneca-troubling Madhi paper. No protection from mild-to-moderate COVID-19 with AstraZeneca in this large clinical trial on young (median age 30 years) people in South Africa. As there were no cases of hospitalization in the study, it remains unclear whether ChAdOx1 nCov-19 may protect against severe infection with the B.1.351 variant. | Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 Mar 16. PubMed: https://pubmed.gov/33725432. Full-text: https://doi.org/10.1056/NEJMoa2102214
