*** The following text is out-of-date.***
For the latest news about COVID-19, please open the COVID Reference homepage.
Published 24 January 2021
The term “Long COVID-19” covers a wide spectrum of symptoms that can occur or persist weeks and even months after an acute infection, not only after severe but also after initially mild courses. Primary symptoms are exhaustion and fatigue, but also dyspnea on exertion, headache and arthralgia, palpitations, concentration disorders and depressive symptoms in previously healthy individuals. The symptoms may fluctuate within hours or days. This chapter summarizes current knowledge (which is still limited, as of the end of January 2021).
“Long Haulers” have to be taken seriously
It was striking how much and how quickly the topic was put on the agenda from the beginning of the pandemic, mainly by patients themselves (Callard 2021). Stories of the so-called “long haulers” were published in social media but also in scientific journals. In early May, the report of the British infectious disease specialist Paul Garner in the BMJ about his seven-week “roller coaster of ill health, extreme emotions and utter exhaustion” gained much attention (Garner 2020). This was followed by many other case reports by medical professionals (Alwan 2020), including that of former UNAIDS Director Peter Piot (Draulens 2020).
Online surveys on persistent cognitive deficits also generated wide circulation (Hampshire 2020), and even if such observations strongly overestimate symptom prevalence, they do provide us with clues about symptoms and distress among long haulers (Goërtz 2020). Indeed, the distress appears to be considerable: among 114 long-haulers (including 32 physicians, almost all treated as outpatients), strong feelings of insecurity, stigmatization, and difficulty being taken seriously were evident (Ladds 2020). In particular, the experience of uncertainty and helplessness were described as stressful. Affected individuals demand that their primary care physician believe their symptoms and show empathy and understanding. Continuous support during recovery and rehabilitation seems to be crucial (Kingstone 2020).
Definition, categories
A generally accepted definition for the syndrome is lacking so far. None of the terms used is a good fit: whether “long”, “chronic”, “ongoing symptomatic”, “post” or “post-acute COVID-19” – they are all incomplete. British experts recommend using the term “post-COVID syndrome” as of 12 weeks post-infection. But as no evidence exists of any particular physiological changes (that predict chronicity) at 12 weeks, according to other experts it would be preferable to use the term long COVID-19 for symptoms of any duration beyond four weeks, as is strongly advocated by people with lived experience of this condition. Moreover, using the prefix “post” implies that acute infection and any active disease process are resolved, which is currently unknown (Sivan 2020). Different time windows of 3, 4, 12, or 24 weeks are discussed. Some authors suggest dividing post-acute manifestations into three categories, namely residual symptoms that persist after recovery from acute infection; organ dysfunction that persists after initial recovery; and new syndromes that develop after initially asymptomatic or mild infection (Amenta 2020). Still others have identified at least four or more distinct entities, including a post-intensive care syndrome (PICS), a post-viral fatigue syndrome, permanent organ damage, and “long-term COVID-19 syndrome” (NIHR 2020, Mahase 2020). To date, no concept has gained broad acceptance. In addition to the lack of definition and valid diagnostic tests, there are several pitfalls and problems that complicate the interpretation of the study data to date (see box).
|
Caveats and problems of studies of “long COVID-19”
|
Pathogenesis
The pathogenesis is certainly multifactorial. Persistent lung damage (as seen in SARS cases) is evident in many patients even after months, especially in severe COVID-19 cases (see below). However, this does not explain the whole syndrome. Speculations and theories about extrapulmonary manifestations involve chronic inflammation, immune dysregulation, sequelae of endotheliitis and coagulopathy, mitochondrial dysfunction – they may all be somewhat correct, and each apply to a subset of cases. Autonomic dysfunction probably also plays a role. It could explain part of the symptoms (dizziness, palpitations, orthostasis) and has been found in a small cohort (Dani 2020). In particular, the main symptom – fatigue, which is somewhat difficult to objectify, remains a puzzle. Overlaps and similarities with the so-called chronic fatigue syndrome (CFS/ME) have been postulated. Of note, a study that looked more closely at fatigue (Townsend 2020) found no association with the severity of COVID-19, but neither was it found with various laboratory markers (blood count, LDH, CRP, IL-6).

Download: 6th Edition – 453 pages
Clinical symptoms
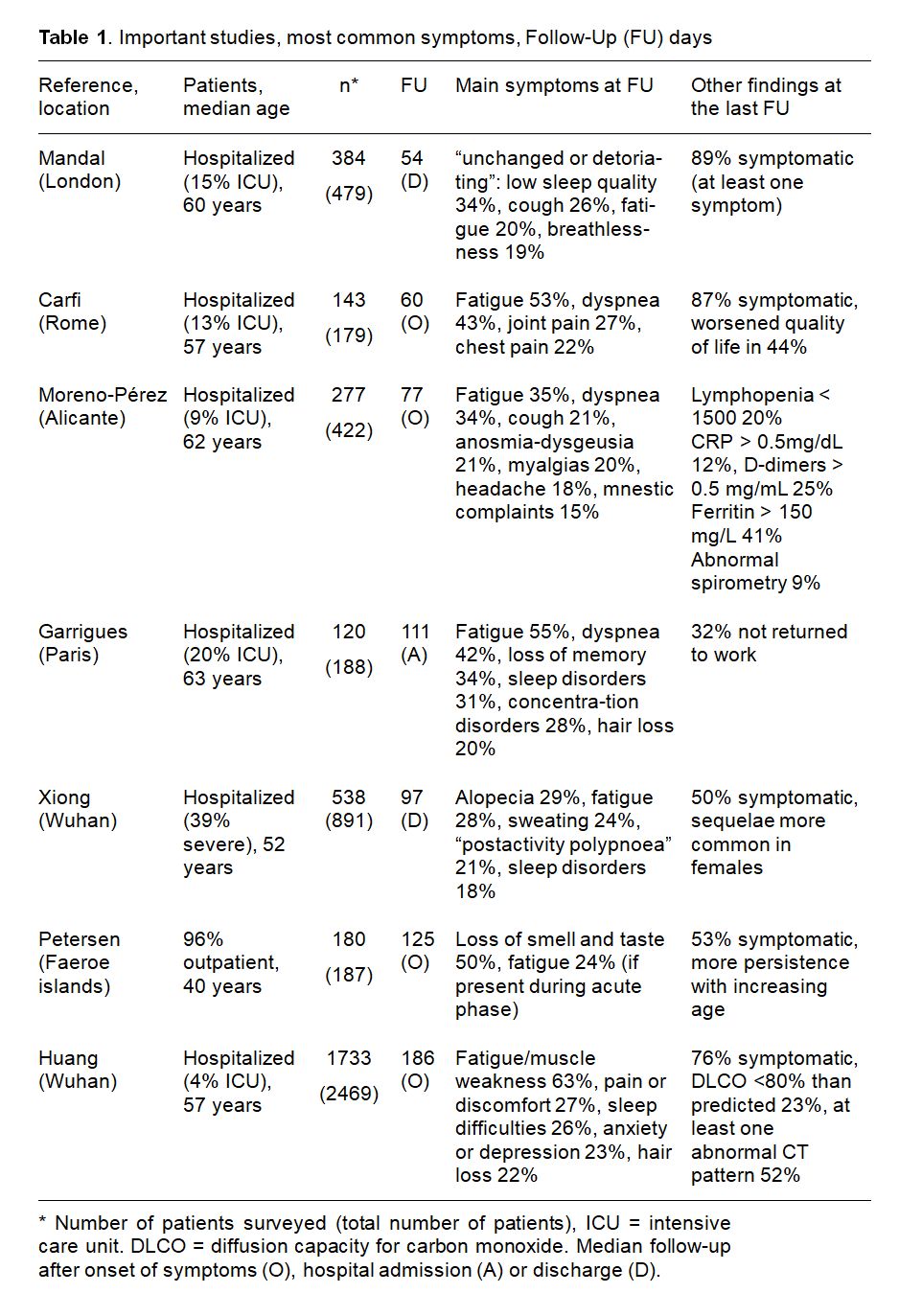
Clinical symptoms and complaints are variable. Beside fatigue and muscle weakness, other common symptoms include shortness of breath, muscle and joint pain, palpitations, loss or alteration of taste and sense of smell, and hair loss. Cognitive problems with deficitis in concentration and memory (“foggy brain”, etc.) are also frequently reported. In the largest studies of clinical symptoms, prevalence varied greatly (Table 1). This may be due to the different time of follow up but also due to very heterogeneous patient populations (severity of COVID-19, age, pre-existing co-morbidities, etc). The by far largest study to date has evaluated 1655 patients from Wuhan (Huang 2021). During acute COVID-19, all patients had been hospitalized (only 4% were treated on an ICU, but 68% had received oxygen). Fatigue and weakness six months later correlated strongly with acute severity of COVID-19, as did anxiety and depression, but also lung diffusion capacity (Huang 2021). Women were more frequently affected.
Persistent symptoms certainly occur even in mild courses. In one well-defined study, 180 (96% of the total) COVID-19 patients from the Faeroe Islands (a North Atlantic archipelago located about halfway between Norway and Iceland) from the first wave were followed: among the almost entirely non-hospitalized cohort, 53% reported persistence of at least one symptom after a mean of 125 days after symptoms onset, 33% reported one or two symptoms and 19% three or more symptoms (Petersen 2020). In the Wuhan study, even of the 439 patients who had not required oxygen in the hospital, 81% reported at least one symptom after 6 months, including 66% fatigue and muscle weakness (Huang 2021). In another large study of 669 patients from Switzerland treated exclusively as outpatients, as many as 32% still had at least one symptom after 6 weeks (Nehme 2020). Long periods of disability are seen even in mild courses (see cases in the box). Of hospitalized patients, almost one-third are unable to work after 3 months (Garrigues 2020, Chopra 2020).
The often highly fluctuating course is striking: in a cross-sectional analysis of 70 “long haulers”, the course of symptoms was intermittent in 43% of the cases, alternating symptom-free intervals of a few days or hours with sudden relapses, often worsening after physical or intellectual exercise (Salmon-Ceron 2020).
Studies with objectifiable tests
A selection of studies that focused on objectifiable tests and parameters is presented below. Again, it is important to consider different follow-up duration, but also the selection of the case population studied. In most studies, there was not only a correlation between the findings and the severity of acute COVID-19 but also a marked improvement over time. However, whether the remaining radiological or pulmonary diffusion abnormalities completely resolve needs to be investigated in future follow-up studies.
Pulmonary function
- Of 145 patients in a prospective study from Germany and Austria (75% hospitalized, 22% in ICU), 41% exhibited persistent symptoms (36% dyspnea) 100 days after COVID-19 onset. CT scans unveiled persistent lung pathologies in 63%, mainly consisting of bilateral ground-glass opacities, without radiological signs of pulmonary fibrosis. However, sequential follow-up evaluations at 60 and 100 days after COVID-19 onset demonstrated a vast improvement of both, symptoms and CT abnormalities over time. One-third displayed an impaired lung function, with a reduced diffusing capacity being the most prominent finding even more than 100 days after COVID-19 diagnosis (Sonnweber 2020).
- In the Wuhan cohort, a subgroup was tested for diffusion capacity for carbon monoxide (DLCO) at 6 months of follow-up. A low DLCO (< 80% of predicted) was found in 50% (48/86) and in 29% (66/228) in those with and without mechanical ventilation during acute COVID-19, respectively (Huang 2021).
- Of 103 consecutively hospitalized patients from Norway (15 ICU cases), about half had exertional dyspnea at three months, a quarter had abnormal CT chest findings, and another quarter had reduced diffusion capacity (Lerum 2020). ICU admission was associated with pathologic CT findings but not with worsened pulmonary function.
- Improvement of CT findings in 99% in 124 patients from Nijmwegen, Netherlands (mostly with mild-moderate disease) after 13 weeks, but residual lesions were seen in 91% of discharged patients, as well as a correlation with diffusion capacity (van den Borst 2020). Cognitive dysfunction was seen in 36%.
- In 113 Swiss patients (66 with severe/critical COVID-19), there was a strong association between acute COVID-19 severity and diffusion capacity at 4 months, as well as between duration of mechanical ventilation and lung function (Guler 2021).
Cardiac MRT
- A total of 100 unselected patients (mean age 49 years, 67 outpatients, including 18 asymptomatic cases), received a cardiac MRI 71 (64-92) days after COVID-19 diagnosis (Puntmann 2020). In total, 36% reported persistent shortness of breath and general fatigue. Of note, MRI showed evidence of “cardiac involvement” in 78% and persistent myocardial inflammation in 60%. This was independent of pre-existing disease, severity of COVID-19, or time of diagnosis.
- In contrast, among 145 young, otherwise healthy students with mild-moderate disease, only two students had an abnormal MRI 14 days after diagnosis (Starekova 2021).
- In 26 competitive athletes (14 asymptomatic, 12 mildly ill), a cardiac MRI was performed 11-53 days after quarantine (Rajpal 2020). Overall, 4/26 (15%) had CMR findings suggestive of myocarditis, and 8/26 (31%) had changes suggestive of previous myocardial injury.
Others
- Physical Fitness: 199 young Swiss recruits had undergone a baseline fitness test (3 months before a major COVID-19 outbreak in the company), including strength measurements and a progressive endurance run. Baseline fitness was compared with a second fitness test performed at a median of 45 days after SARS-CoV-2 diagnosis. Three groups were formed: “symptomatic COVID-19” (n = 68, all mild-moderate), asymptomatic cases (n = 77), and symptom-free without evidence for infection (n = 54). The strength tests were comparable between the groups. However, there was a significant decrease in VO2 max in the symptomatic cases. Approximately 19% had a decrease in VO2 max of more than 10%, whereas none of the uninfected showed such a decrease (Crameri 2020).
COVID Reference resources:
Home / Today | Top 10: Last 21 days | Top 10 Archive | Download | Epidemiology | Transmission | Prevention | Virology | Vaccines | Diagnosis | Clinical Manifestations | Treatment | Severe COVID-19 | Comorbidities / Special Populations | Pediatrics| Timeline | Preface
Monitoring, treatment options
As early as August 2020, a preliminary guideline for the treatment of “long COVID-19” was published in the British Medical Journal (Greenhalgh 2020). After excluding serious ongoing complications or comorbidities, the recommendation was to manage patients “pragmatically and symptomatically with an emphasis on holistic support while avoiding over-investigation”. It was noted that “many patients recover spontaneously (if slowly) with holistic support, rest, symptomatic treatment, and gradual increase in activity”.
According to the authors, blood tests should “be ordered selectively and for specific clinical indications after a careful history and examination; the patient may not need any”. In the largest and longest study to date from Wuhan, however, 35% of the patients showed a decreased glomerular filtration rate (GFR). Unexpectedly, 13% (107 of 822) of those who did not develop acute kidney injury during their hospital stay and presented with normal renal function, based on estimated GFR during the acute phase, exhibited a decline in eGFR (< 90 mL/min per 1,73 m2) at 6 months of follow-up (Huang 2021). It seems therefore reasonable to monitor renal function at least once in long COVID-19 cases.
Fortunately, new onset diabetes mellitus and thrombosis were extremely rare in the Wuhan cohort study (Huang 2021). From our point of view, the control of blood glucose or D-dimers (as well as the use anticoagulation as suggested by some experts) does not seem to be necessary. This also applies to inflammatory parameters which can be slightly elevated in a considerable proportion of patients even after months (Moreno-Pérez 2021). These remain without consequences.
|
Case 1 51-year-old resident surgeon with mild-to-moderate COVID-19 (outpatient, no oxygen, but severe headache, nausea, no fever, 2-3 days of dyspnea). Unable to work (including quarantine) for 24 days. Reintegration to work (starting with 3 hrs) after another 35 days. Current comment (after 90 days): “Still fluctuating days of strongest exhaustion, only very gradual recovery. I am glad to be able to get my work done. However, every day, I still have to sleep 1-3 hours after work. Improvement is slow. I was already jogging again, but still feelings of fatigue rapidly occuring, like ‘plug out’”. Case 2 44-year-old psychiatrist with mild-to-moderate COVID-19 (outpatient, no oxygen). Beyond the two weeks of quarantine, there is dyspnea on exertion, severe exhaustion and cephalgia, concentration disorders, mild anxious-depressive symptoms (self-assessment) and anosmia/ageusia. Another 21 days of incapacity for work (total 35), followed by slow reintegration to work over a further 42 days. Current comment (after 90 days): “Now just the second week of normal working hours. No longer headaches on all days. Fatigue still evident, severe on some days. Sense of smell and taste still not present. Sport is not to be thought of.” |
A light endurance training (including walking or Pilates, increasing intensity only very gradually) may be useful, as may relaxation techniques. Gradual re-integration into working life, as in the two case studies, is often helpful. Patience, empathy and a cautious (not too ambitious) goal-setting are required. Especially in severe cases, inpatient rehabilitation may be useful, preferably in multidisciplinary settings; some experiences have already been published (Puchner 2021, Brigham 2021).
Numerous clinical therapeutic trials of long COVID are ongoing or planned, including those with steroids, but also different drugs such as naltrexone, leronlimab, montelukast, or deupirfenidone. Up to now, no results have been published. In addition, many large prospective clinical trials will study long haulers prospectively and in a standardized manner in order to better understand the long-term effects on lung function, the cardiovascular system, hematologic parameters, renal function, and many other organ systems. For example, in the United Kingdom, the Post-Hospitalisation COVID-19 Study (PHOSP-COVID) will follow 10.000 patients for a year, analysing clinical factors such as blood tests and scans, and collecting data on biomarkers. Finally, existing cohort studies have also refocused and integrated COVID-19 aspects in their study designs (Abbott 2020). These studies will have the advantage of having a control group (those who do not become infected).
Conclusion
A relevant number of people with COVID-19 are physically and psychologically impaired up to at least 6 months after diagnosis. Women appear to be more frequently affected. The studies to date are cause for concern; on the other hand, it must be kept in mind that retrospective studies are subject to numerous biases and tend to overestimate the disease burden. However, it has become evident that “long COVID-19” needs to gain even more attention in the current discussion. Those who now emphasize the collateral damage and costs of lockdown should also consider the consequences of long COVID-19 or the often very slow convalescence of many cases. Those who discuss potential long-term damage from vaccines should also consider the potential long-term damage from COVID-19. “Long haulers” need to be taken seriously. Given the high numbers of SARS-CoV-2 infections world-wide, all practicing physicians and outpatient clinics will be dealing with this syndrome in the future.
References
Abbott A. Thousands of people will help scientists to track the long-term health effects of the coronavirus crisis. Nature NEWS 582, 326 (2020). Full-text: https://doi.org/10.1038/d41586-020-01643-8
Alwan NA. A negative COVID-19 test does not mean recovery. Nature NEWS August 11, 2020. Full-text: https://www.nature.com/articles/d41586-020-02335-z
Amenta EM, Spallone A, Rodriguez-Barradas MC, et al. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect Dis. 2020 Oct 21;7(12):ofaa509. PubMed: https://pubmed.gov/33403218. Full-text: https://doi.org/10.1093/ofid/ofaa509
Brigham E, O’Toole J, Kim SY, et al. The Johns Hopkins Post-Acute COVID-19 Team (PACT): A Multidisciplinary, Collaborative, Ambulatory Framework Supporting COVID-19 Survivors. Am J Med. 2021 Jan 11:S0002-9343(20)31174-8. PubMed: https://pubmed.gov/33444589. Full-text: https://doi.org/10.1016/j.amjmed.2020.12.009
Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021 Jan;268:113426. PubMed: https://pubmed.gov/33199035. Full-text: https://doi.org/10.1016/j.socscimed.2020.113426
Carfì A, Bernabei R, Landi F, for the Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603-605. PubMed: https://pubmed.gov/32644129. Full-text: https://doi.org/10.1001/jama.2020.12603
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2020 Nov 11:M20-5661. PubMed: https://pubmed.gov/33175566. Full-text: https://doi.org/10.7326/M20-5661
Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021 Jan 16;397(10270):173-175. PubMed: https://pubmed.gov/33428868. Full-text: https://doi.org/10.1016/S0140-6736(21)00039-8
Crameri GAG, Bielecki M, Züst R, Buehrer TW, Stanga Z, Deuel JW. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020 Sep;25(36):2001542. PubMed: https://pubmed.gov/32914744. Full-text: https://doi.org/10.2807/1560-7917.ES.2020.25.36.2001542
Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2020 Nov 26:clinmed.2020-0896. PubMed: https://pubmed.gov/33243837. Full-text: https://doi.org/10.7861/clinmed.2020-0896
Draulens D. ‘Finally, a virus got me.’ Scientist who fought Ebola and HIV reflects on facing death from COVID-19. Sciencemag May 8, 2020. https://www.sciencemag.org/news/2020/05/finally-virus-got-me-scientist-who-fought-ebola-and-hiv-reflects-facing-death-covid-19
Garner P. For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion. BMJ Opinion May 5, 2020. https://blogs.bmj.com/bmj/2020/05/05/paul-garner-people-who-have-a-more-protracted-illness-need-help-to-understand-and-cope-with-the-constantly-shifting-bizarre-symptoms/
Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020 Aug 25:S0163-284 PubMed: https://pubmed.gov/32853602. Full-text: https://doi.org/10.1016/j.jinf.2020.08.029
Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020 Oct 26;6(4):00542-2020. PubMed: https://pubmed.gov/33257910. Full-text: https://doi.org/10.1183/23120541.00542-2020.
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020 Aug 11;370:m3026. PubMed: https://pubmed.gov/32784198. Full-text: https://doi.org/10.1136/bmj.m3026
Guler SA, Ebner L, Beigelman C, et al. Pulmonary function and radiological features four months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021 Jan 8:2003690. PubMed: https://pubmed.gov/33419891. Full-text: https://doi.org/10.1183/13993003.03690-2020
Hampshire A, Trender W, chamberlain SR, et al. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N=84,285 online study. Medrxv, doi: https://doi.org/10.1101/2020.10.20.20215863
Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2020 Dec 21:1-23. PubMed: https://pubmed.gov/33347790. Full-text: https://doi.org/10.1080/10408363.2020.1860895
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220-232. PubMed: https://pubmed.gov/33428867. Full-text: https://doi.org/10.1016/S0140-6736(20)32656-8
Kingstone T, Taylor AK, O’Donnell CA, et ak. Finding the ‘right’ GP: a qualitative study of the experiences of people with long-COVID. BJGP Open. 2020 Dec 15;4(5):bjgpopen20X101143. PubMed: https://pubmed.gov/33051223. Full-text: https://doi.org/10.3399/bjgpopen20X101143
Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020 Dec 20;20(1):1144. PubMed: https://pubmed.gov/33342437. Full-text: https://doi.org/10.1186/s12913-020-06001-y
Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J. 2020 Dec 10:2003448. PubMed: https://pubmed.gov/33303540. Full-text: https://doi.org/10.1183/13993003.03448-2020
Mahase E. Long covid could be four different syndromes, review suggests. BMJ. 2020 Oct 14;371:m3981. PubMed: https://pubmed.gov/33055076. Full-text: https://doi.org/10.1136/bmj.m3981
Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020 Nov 10:thoraxjnl-2020-215818. PubMed: https://pubmed.gov/33172844. Full-text: https://doi.org/10.1136/thoraxjnl-2020-215818
Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 Syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021 Jan 12:S0163-4453(21)00009-8. PubMed: https://pubmed.gov/33450302. Full-text: https://doi.org/10.1016/j.jinf.2021.01.004
Nehme M, Braillard O, Alcoba G, et al. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Ann Intern Med. 2020 Dec 8:M20-5926. PubMed: https://pubmed.gov/33284676. Full-text: https://doi.org/10.7326/M20-5926
NIHR 2020. Living with covid-19. A dynamic review of the evidence around ongoing covid-19 symptoms (often called long covid). https://evidence.nihr.ac.uk/themedreview/living-with-covid19
Nunn AV, Guy GW, Brysch W. et al. SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing. Immun Ageing 17, 33 (2020). https://doi.org/10.1186/s12979-020-00204-x
Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands – a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 Nov 30:ciaa1792. PubMed: https://pubmed.gov/33252665. Full-text: https://doi.org/10.1093/cid/ciaa1792
Puchner B, Sahanic S, Kirchmair R, et al. Beneficial effects of multi-disciplinary rehabilitation in post-acute COVID-19 – an observational cohort study. Eur J Phys Rehabil Med. 2021 Jan 15. PubMed: https://pubmed.gov/33448756. Full-text: https://doi.org/10.23736/S1973-9087.21.06549-7
Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance (CMR) Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Jul 27:e203557. PubMed: https://pubmed.gov/32730619. Full-text: https://doi.org/10.1001/jamacardio.2020.3557
Rajpal S, Tong MS, Borchers J, et al. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2020 Sep 11:e204916. PubMed: https://pubmed.gov/32915194. Full-text: https://doi.org/10.1001/jamacardio.2020.4916
Raveendran AV. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr. 2020 Dec 15;15(1):145-146. PubMed: https://pubmed.gov/33341598. Full-text: https://doi.org/10.1016/j.dsx.2020.12.025
Salmon-Ceron D, Slama D, De Broucker T, et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: A cross-sectional study. J Infect. 2020 Dec 4:S0163-4453(20)30762-3. PubMed: https://pubmed.gov/33285216. Full-text: https://doi.org/10.1016/j.jinf.2020.12.002
Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020 Dec 23;371:m4938. PubMed: https://pubmed.gov/33361141. Full-text: https://doi.org/10.1136/bmj.m4938
Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2020 Dec 10:2003481. PubMed: https://pubmed.gov/33303539. Full-text: https://doi.org/10.1183/13993003.03481-2020
Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging. JAMA Cardiol. 2021 Jan 14. PubMed: https://pubmed.gov/33443537. Full-text: https://doi.org/10.1001/jamacardio.2020.7444
Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020 Nov 9;15(11):e0240784. PubMed: https://pubmed.gov/33166287. Full-text: https://doi.org/10.1371/journal.pone.0240784. eCollection 2020
van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020 Nov 21:ciaa1750. PubMed: https://pubmed.gov/33220049. Full-text: https://doi.org/10.1093/cid/ciaa1750
Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021 Jan;27(1):89-95. PubMed: https://pubmed.gov/32979574. Full-text: https://doi.org/10.1016/j.cmi.2020.09.023