9 November
Aguas R, Bharath A, White LJ, et al. Potential global impacts of alternative dosing regimen and rollout options for the ChAdOx1 nCoV-19 vaccine. Nature Commun November 4, 2021. https://www.nature.com/articles/s41467-021-26449-8
Using data from clinical trials, an individual-based model was constructed to predict its 6-month population-level impact. The main result: in scenarios where the availability of vaccine is insufficient for high-risk groups to receive two doses, administration of a single dose is optimal, even when vaccine efficacy after one dose is just 75% of the two doses.
Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun November 4, 2021, 12, 6379. https://www.nature.com/articles/s41467-021-26672-3
Leveraging the centralized computer database of Maccabi Healthcare Services, the authors assessed the correlation between time-from-vaccine and incidence of breakthrough infection between June 1 and July 27, 2021. There was a significant correlation between time-from-vaccine and afforded protection against SARS-CoV-2 infection. The risk for breakthrough infection was significantly higher for longer time-from-vaccine recipients compared with those who were vaccinated more recently, with an additional trend for higher risk for hospitalization among the longer time-from-vaccine recipients.
8 November
McNamara LA, Wiegand RE, Burke RM, et al. Estimating the early impact of the US COVID-19 vaccination programme on COVID-19 cases, emergency department visits, hospital admissions, and deaths among adults aged 65 years and older: an ecological analysis of national surveillance data. Lancet November 03, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02226-1/fulltext
Vaccines are working at the population level. The initial phases of the US COVID-19 vaccination program were associated with reductions in COVID-19 cases, emergency department visits, and hospital admissions among US adults aged 65 years and older. COVID-19 deaths also declined.
7 November
Tenforde M, Self WH, Adams K, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA November 4, 2021. https://jamanetwork.com/journals/jama/fullarticle/2786039?resultClick=1
In this case-control study that included 4513 hospitalized adults in 18 US states, hospitalization for a COVID-19 diagnosis compared with an alternative diagnosis was associated with an adjusted odds ratio (aOR) of 0.15 for full vaccination with an authorized or approved mRNA COVID-19 vaccine.
Eliakim-Raz N, Leibovici-Weisman Y, Stemmer A, et al. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥60 Years. JAMA November 5, 2021 https://jamanetwork.com/journals/jama/fullarticle/2786096?resultClick=1
This study on 97 adults aged 60 years and older found that a third BNT162b2 dose was associated with significantly increased IgG titers after 10 to 19 days, with no major adverse events.
Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021 Nov;41:101158. doi: 10.1016/j.eclinm.2021.101158. Epub 2021 Oct 17. https://pubmed.ncbi.nlm.nih.gov/34693234/
In this large cohort from Israel, antibody response to the Pfizer-BioNTech vaccine was highly variable among different immune deficiencies. RBD-IgG antibodies were detected in 154/156 (98.7%) of patients with HIV, but only in 96/188 (51.0%) of those with chronic lymphocytic leukemia/non-Hodgkin’s lymphoma and in 50/110 (45.5%) of patients following kidney transplantation.
6 November
Woodworth KR, Moulia D, Collins JP, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Children Aged 5–11 Years — United States, November 2021. MMWR 5 November 2021. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7045e1.htm?s_cid=mm7045e1_w#suggestedcitation
Interim recommendation for use of the Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years in the United States for prevention of COVID-19.
5 November
Bosch W, Cowart JB, Bhakta S, et al. COVID-19 Vaccine-Breakthrough Infections Requiring Hospitalization in Mayo Clinic Florida through August 2021. Clinical Infectious Diseases 2 November 2021, ciab932, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab932/6415962
Of 1089 breakthrough infections occurring after May 2, 2021, 12% (n=126) required hospitalization. When compared to unvaccinated COVID-19 admissions, vaccine breakthrough admissions were older in age (mean 69.1 vs. 59.6 years, p<0.001), more likely to be immunocompromised (33.3% vs. 14.8%); p<0.001) and had a higher COVID-19 Complication Risk score. In addition, the vaccinated cohort was more likely to have diabetes, hypertension, coronary artery disease and chronic kidney disease.
Ashrani AA, Crusan DJ, Petterson T, et al. Age- and Sex-Specific Incidence of Cerebral Venous Sinus Thrombosis Associated With Ad26.COV2.S COVID-19 Vaccination. JAMA Intern Med November 1, 2021. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2785610?resultClick=1
Most CVST events occurred within 15 days after vaccination. The highest risk was among women aged 30 to 49 years, but the absolute CVST risk was still low in this group (up to 29.5 per 100 000 PY among women aged 40-49 years).
1 November
Lanini S, Capone S, Antinori A, et al. GRAd-COV2, a gorilla adenovirus-based candidate vaccine against COVID-19, is safe and immunogenic in younger and older adults. Science Translational Medicine 26 October 2021. https://www.science.org/doi/10.1126/scitranslmed.abj1996
A COVID-19 vaccine based on a replication-defective gorilla adenovirus expressing the stabilized pre-fusion SARS-CoV-2 spike protein, named GRAd-COV2. The safety and immunogenicity of a single-dose regimen of this vaccine were good in this Phase I, dose-escalation, open-label trial in 90 healthy participants. (Without a doubt, Joshua would refuse a vaccine derived “from a threatened species”).
31 October
Barda N, Dagan N, Cohen C. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet October 29, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02249-2/fulltext
Older than 40? This is currently the best data for a third jab. In this large study, the third dose and matched control groups each included 728,321 individuals. Participants had a median age of 52 years (IQR 37–68). Evaluated at least 7 days after the third dose, compared with receiving only two doses at least 5 months previously, was estimated to be 93% (231 vs 29 events) for vaccine effectiveness, 92% (157 vs 17 events) for severe disease, and 81% (44 vs 7 events) for COVID-19-related death. Effectiveness was seen in all age groups above 40. Only in those aged 16–39 years, the rate of these severe outcomes was too small for any meaningful estimation of effectiveness of the booster.
Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines — United States, 2021. MMWR Morb Mortal Wkly Rep. ePub: 29 October 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7044e2.htm?s_cid=mm7044e2_w
Detailed recommendations for a third jab in moderately to severely immunocompromised persons and in persons with certain underlying conditions.
Bozio CH, Grannis SJ, Naleway AL, et al. Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity — Nine States, January–September 2021. MMWR Morb Mortal Wkly Rep. ePub: 29 October 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7044e1.htm?s_cid=mm7044e1_w
Vaccine is better than previous infection. In this US network, laboratory-confirmed SARS-CoV-2 infection was identified among 324 (5.1%) of 6328 fully vaccinated persons and among 89 of 1020 (8.7%) unvaccinated, previously infected persons. The adjusted odds of laboratory-confirmed COVID-19 among unvaccinated adults with previous SARS-CoV-2 infection were 5.49-fold higher than the odds among fully vaccinated recipients of an mRNA COVID-19 vaccine who had no previous documented infection (95% confidence interval: 2.75–10.99).
30 October
Li L, Robinson LB, Patel R, et al. Association of Self-reported High-Risk Allergy History With Allergy Symptoms After COVID-19 Vaccination. JAMA Netw Open October 26, 2021;4(10):e2131034. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2785466?resultClick=1
In this large study on individuals with vs without a history of high-risk allergy more allergic reactions were reported after receiving dose 1 or 2 of the vaccine (11.6% [n = 55] vs 4.7% [n = 2461]). However, reported allergy symptoms did not impede the completion of the 2-dose vaccine protocol.
Woldesmekel BA, Garliss CC, Blankson JN. mRNA Vaccine-Elicited SARS-CoV-2-Specific T cells Persist at 6 Months and Recognize the Delta Variant. Clinical Infectious Diseases October 25, 2021, ciab915, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab915/6409907
This small study found only a modest decline in the frequency of these T cells at 6 months. Moreover, there was a robust expansion in response to antigen and recognition of spike peptides from the Delta variant, indicating that booster shots should successfully increase the frequency of SARS-CoV-2-specific T cells in circulation.
28 October
Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med October 25, 2021. https://www.nature.com/articles/s41591-021-01556-7
Although this study found an increased risk of neurological complications in those who received COVID-19 vaccines, the risk is greater from COVID-19. There was a substantially higher risk of all neurological outcomes in the 28 days after a positive SARS-CoV-2 test including Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18).
27 October
Harder T, Külper-Schiek W, Reda S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26(41):pii=2100920. https://doi.org/10.2807/1560-7917.ES.2021.26.41.2100920
The main conclusion of this review: COVID-19 vaccines approved in the EU have a moderate to high effectiveness against mild to moderate forms of SARS-CoV-2 infections caused by the Delta variant, while VE against severe disease and hospitalization remains high to very high.
Nordström P, Ballin M, Nordström A, et al. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet Regional Health October 18, 2021. https://www.sciencedirect.com/science/article/pii/S2666776221002350?via%3Dihub
This study compared >100,000 individuals who received heterologous AZ/BioNTech or Moderna and 430,100 individuals that received homologous AZ/BioNTech prime-boost vaccination. Heterologous vaccination showed 67% to 79% effectiveness against symptomatic COVID-19, higher than the 50% effectiveness from homologous vaccination.
26 October
Xu S, Huang R, Sy LS, et al. COVID-19 Vaccination and Non–COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep. ePub: 22 October 2021. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7043e2.htm?s_cid=mm7043e2_w
There is no increased risk for mortality among COVID-19 vaccine recipients. During December 2020–July 2021, COVID-19 vaccine recipients had lower rates of non–COVID-19 mortality than did unvaccinated persons after adjusting for age, sex, race and ethnicity, and study site.
Hillson K, Clemens SC, Madhi SA, et al. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. https://doi.org/10.1016/S0140-6736(21)02282-0
No, there is no fertility issue. Fertility was unaffected among 9755 participants in RCTs. In addition, the rate of miscarriage was not higher in the ChAdOx1 nCoV-19 group.
24 October
Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 6566, October 22, 2021. https://www.science.org/doi/10.1126/science.abj9853
A smaller jab does the job. Studying 35 vaccinated subjects 7 months out from the initial immunization, the authors found that two-dose 25-mg mRNA-1273 vaccination (Moderna, instead of 100 mg) generated immune memory against spike comparable to that of SARS-CoV-2 infection for antibodies and T-cells.
23 October
Pfizer. Press release, October 21, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-phase-3-trial-data-showing
Time for a third jab? Unfortunately, this has been published only as a press release. In this phase 3 RCT evaluating the efficacy and safety of a 30-µg booster dose of the Pfizer-BioNTech COVID-19 Vaccine in more than 10,000 individuals 16 years of age and older, participants who previously completed the primary two-dose series were randomized 1:1 to receive either a 30-µg booster dose (the same dosage strength as those in the primary series) or placebo. The median time between second dose and the booster dose or placebo was 11 months. From day 7, there were 5 cases of COVID-19 in the booster group and 109 cases in the non-boosted group. The observed relative vaccine efficacy was 95.6% (95% CI: 89.3, 98.6).
22 October
Reis BY, Barda N, Leshchinsky M. Effectiveness of BNT162b2 Vaccine against Delta Variant in Adolescents. NEJM October 2021, https://www.nejm.org/doi/full/10.1056/NEJMc2114290?query=featured_home
In 94,354 vaccine recipients from Israel between the ages of 12 and 18 years who were successfully matched with 94,354 unvaccinated controls, effectiveness against documented SARS-CoV-2 infection was 90% (95% CI: 88% – 92%) on days 7 to 21 after the second dose.
Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years — United States, June–September 2021. MMWR Morb Mortal Wkly Rep. ePub: 19 October 2021.
Same findings from the US. Among hospitalized patients aged 12–18 years, vaccine effectiveness of 2 doses of the Pfizer/BioNTech vaccine against COVID-19 hospitalization during June–September 2021, was 93% (95% CI: 83%–97%).
Chin ET, Leidner D, Zhang Y, et al. Effectiveness of the mRNA-1273 Vaccine during a SARS-CoV-2 Delta Outbreak in a Prison. NEJM October 20, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2114089?query=featured_home
The Sierra Conservation Center (SCC) in Jamestown, California, is a low-to-medium security prison for men. During an outbreak among residents (468 were fully vaccinated and 359 were unvaccinated), the estimated vaccine effectiveness was 56.6% against infection and 84.2% against symptomatic infection.
Sheikh A, Robertson C, Taylor B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. NEJM October 20, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2113864?query=featured_home
Overall, vaccine effectiveness against death from the Delta variant 14 or more days after the second vaccine dose was 90% for BNT162b2 and 91% for ChAdOx1 nCoV-19.
Sciascia S, Costanzo P, Radin M, et al. Safety and tolerability of mRNA COVID-19 vaccines in people with antiphospholipid antibodies. Lancet Rheumatology, October 20, 2021. https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(21)00320-9/fulltext
No severe problems with vaccines among 102 patients (52 with a diagnosis of antiphospholipid syndrome and 50 with antiphospholipid antibodies without clinical features).
Waltz E. COVID vaccine makers brace for a variant worse than Delta. Nature News October 20, 2021. https://www.nature.com/articles/d41586-021-02854-3
Nice article by Emily Waltz, summarizing how companies are updating vaccines and testing them on people to prepare for whatever comes next in the pandemic.
21 October
Rovida F, Cassaniti I, Paolucci S, et al. SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers. Nat Commun 12, 6032, October 15, 2021. https://www.nature.com/articles/s41467-021-26154-6
All 33 vaccine breakthrough infections were asymptomatic or symptomatic, mostly with few and mild symptoms like rhinitis.
Dhaval D, Friedson AI, Hansen B, et al. Association Between Statewide COVID-19 Lottery Announcements and Vaccinations. JAMA Health Forum, October 15, 2021. https://jamanetwork.com/journals/jama-health-forum/fullarticle/2785288?resultClick=1
Lottery-style incentives may be less effective than those that definitely pay.
18 October
Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, published 15 October. Full text: https://www.cell.com/cell/fulltext/S0092-8674(21)01221-6
Antibody levels after two doses of the BioNTech/Pfizer vaccine are higher after an extended dosing interval in infection-naïve participants compared with the 3-4 week dosing interval used in the licensing studies. The extended regimen also enriches for virus-specific CD4+ T cells expressing IL-2.
17 October
Cohn BA Cirillo PM, Murphy CC, et al. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans, February 1, 2021 to August 13, 2021. medRxiv 2021, posted 14 October. Full text: https://doi.org/10.1101/2021.10.13.21264966
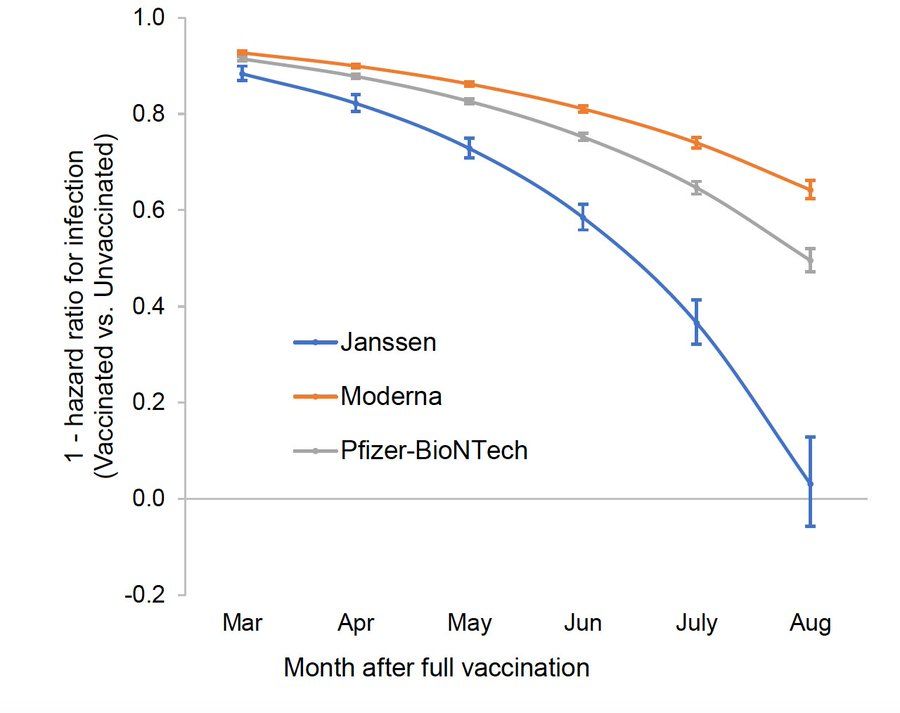
Collier AY, Yu J, McMahan K, et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N Engl J Med. 2021 Oct 15. PubMed: https://pubmed.gov/34648703. Full text: https://doi.org/10.1056/NEJMc2115596
Highly interesting data from a small number of individuals who received either the BioNTech/Pfizer vaccine (n = 31) or the Moderna (n = 22) or the Johnson & Johnson (n = 8) vaccines. At 8 months of follow-up, the median live virus neutralizing antibody titers, pseudovirus neutralizing antibody titers, and RBD-specific binding antibody titers elicited by the BioNTech/Pfizer vaccine were lower than the peak titers by a factor of 34, 4, and 29, respectively. The Johnson & Johnson vaccine never reached titers as high as for the mRNA vaccines; however, at 8 months, the J&J titers were similar to those at week 4. Bigger cohorts needed to confirm these data.
Rubin R. Trying to Block SARS-CoV-2 Transmission With Intranasal Vaccines. JAMA. 2021 Oct 14. PubMed: https://pubmed.gov/34647956. Full text: https://doi.org/10.1001/jama.2021.18143
What appears to be promising in animals doesn’t always pan out in humans.
16 October
Mallapaty S. China’s COVID vaccines have been crucial — now immunity is waning. Nature 2021, published 14 October. Full text: https://www.nature.com/articles/d41586-021-02796-w
“Billions of shots of China’s CoronaVac and Sinopharm vaccines have been given globally, but studies have questioned the length of protection they offer.”
CDC 202110. Rates of COVID-19 Cases and Deaths by Vaccination Status. Centers for Disease Control 2021. Link: https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status
The CDC is now posting data on vaccination status for hospitalization and death, and by age.
15 October
Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS-CoV-2 Booster Vaccinations: Preliminary Report. medRxiv 2021, posted 13 October. Full text: https://doi.org/10.1101/2021.10.10.21264827
The authors evaluated homologous and heterologous booster vaccination in persons who had completed a full COVID-19 vaccine regimen at least 12 weeks earlier. Of 458 individuals, 154 received the BioNTech/Pfizer, 154 received the Moderna, and 150 received the Johnson & Johnson booster vaccine. The authors observed a substantial increase in neutralizing antibody titers in all study participants irrespective of booster and primary vaccine series.
Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021 Oct 14;385(16):1474-1484. PubMed: https://pubmed.gov/34320281. Full text: https://doi.org/10.1056/NEJMoa2109072
An analysis of breakthrough infections among 39 fully vaccinated health care workers during the 4-month period after the second vaccine dose. Neutralizing antibody titers in case patients during the peri-infection period were lower than those in matched uninfected controls, and higher peri-infection neutralizing antibody titers were associated with lower infectivity (higher Ct values). Most breakthrough cases were mild or asymptomatic, although 19% had persistent symptoms (> 6 weeks).
Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N Engl J Med. 2021 Oct 14;385(16):1533-1535. PubMed: https://pubmed.gov/34496196. Full text: https://doi.org/10.1056/NEJMc2113891
Is there a risk of spontaneous abortion after receipt of an mRNA COVID-19 vaccine either before conception (30 days before the first day of the last menstrual period through 14 days after) or during pregnancy? It doesn’t seem so. Among 2456 participants enrolled in the CDC v-safe COVID-19 pregnancy registry, the cumulative risks of spontaneous abortion were within the expected range.
14 October
Marcotte H, Piralla A, Zuo F, et al. Immunity to SARS-CoV-2 up to 15 months after infection. bioRxiv 2021, posted 11 October. Full text: https://doi.org/10.1101/2021.10.08.463699
More about the longevity of immunity of SARS-CoV-2 immunity. The data “suggest that antiviral specific immunity especially memory B cells in COVID-19 convalescent patients is long-lasting, but some variants of concern, including the fast-spreading Delta variant, may at least partially escape the neutralizing activity of plasma antibodies.”
13 October
Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021 Oct 11. PubMed: https://pubmed.gov/34634791. Full text: https://doi.org/10.1038/s41586-021-04085-y
Analysis of plasma neutralization using 16 SARS-CoV-2 variants. The Beta (B.1.351) and Gamma (P.1) strains showed the greatest reduction, followed by the Delta (B.1.617.2) and Alfa (B.1.1.7) strains. Plasma from previously infected vaccinated individuals produced very high neutralizing antibodies against most variants of concern.
Saiag E, Goldshmidt H, Sprecher E, Ben-Ami R, Bomze D. Immunogenicity of a BNT162b2 vaccine booster in health-care workers. Lancet Microbe 2021, published 11 October. Full text: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00272-X/fulltext
The authors report on 346 health care workers (median age: 67 years) of a hospital in Tel Aviv, Israel, who received a third dose of the BioNTech/Pfizer vaccine. “The median (…) SARS-CoV-2 IgG index at baseline was 3.67 (IQR 2.00–7.10), and increased to > 150 (the upper limit of quantification) in 95.7% of vaccine recipients.”
Mahase E. Covid-19: Antibody levels fall after second Pfizer dose, but protection against severe disease remains, studies indicate. BMJ. 2021 Oct 11;375:n2481. PubMed: https://pubmed.gov/34635474. Full text: https://doi.org/10.1136/bmj.n2481
Even falling antibody levels protect against severe COVID-19. A short comment on the two NEJM papers by Levin and Chemaitelly.
11 October
Mallapaty S. Heart-inflammation risk from Pfizer COVID vaccine is very low. Nature 2021, published 8 October. Full text: https://www.nature.com/articles/d41586-021-02740-y
Three days ago, we presented two papers about myocarditis after COVID-19 vaccination. Find a short summary in this Nature news feature.
Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. JAMA. 2021 Oct 7. PubMed: https://pubmed.gov/34617967. Full text: https://doi.org/10.1001/jama.2021.16496
February 2021 to July 2021, 130 cases of presumptive Guillain-Barré Syndrome (GBS) were reported within the US Vaccine Adverse Event Reporting System following vaccination with the Johnson & Johnson vaccine. The overall estimated observed to expected rate ratio was 4.18, corresponding to an absolute rate increase of 6.36 per 100,000 person-years.
10 October
Clemens SAC, Folegatti PM, Emary KRW, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nat Commun. 2021 Oct 6;12(1):5861. PubMed: https://pubmed.gov/34615860. Full text: https://doi.org/10.1038/s41467-021-25982-w
The authors report that the AstraZeneca vaccine protects against various SARS-CoV-2 variants in Brazil, even those that have the spike protein mutation E484K: Zeta (P.2), 69%; B.1.1.28, 73%. The AstraZeneca vaccine also provided 95% protection against hospitalization due to COVID-19.
9 October
Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021 Oct 4:S0140-6736(21)02183-8. PubMed: https://pubmed.gov/34619098. Full text: https://doi.org/10.1016/S0140-6736(21)02183-8
Retrospective study, n = 3,436,957. Effectiveness of the BioNTech/Pfizer vaccine against SARS-CoV-2 infections declined from 88% during the first month after full vaccination to 47% after 5 months. The good news: vaccine effectiveness against hospital admissions for infections with the Delta variant for all ages was 93% up to 6 months.
Seror R, Camus M, Salmon JH, et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry. Rheumatology 2021, published 6 October. Full text: https://doi.org/10.1016/S2665-9913(21)00314-3
In 113 patients on disease modifying anti-rheumatic drugs (87% had rheumatoid arthritis and 13% had psoriatic arthritis), such as such as JAK inhibitors, the overall response rate to vaccination was 88%. Non-responders were older than responders. The rate of non-response was higher with upadacitinib (seven [26%] of 27 patients) than with baricitinib (five [9%] of 56) or tofacitinib (one [3%] of 30).
30 September
Hause AM, Baggs J, Gee J, et al. Safety Monitoring of an Additional Dose of COVID-19 Vaccine — United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep 28 September 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7039e4.htm?s_cid=mm7039e4_w
Among 12,591 registrants who completed a health check-in survey after all 3 doses of an mRNA, adverse reactions after dose 3 were similar to those after dose 2.
Glatman-Freedman A, Hershkovitz Y, Kaufman Z, et al. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis September 27, 2021. https://wwwnc.cdc.gov/eid/article/27/11/21-1886_article
Nationwide retrospective cohort study, showing a crude vaccine effectiveness against laboratory-confirmed SARS-CoV-2 infection of 88% in the fourth week among adolescents.
29 September
Sánchez van Kammen M, Aguiar de Sousa D, Poli S, et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. JAMA Neurology September 28, 2021. https://jamanetwork.com/journals/jamaneurology/fullarticle/2784622?resultClick=1
International registry of 116 CVST cases occurring after SARS-CoV-2 vaccination, among them 78 cases with CVST-TTS (76 had received ChAdOx1 nCov-19 vaccine from AstraZeneca). These patients had a clinical profile distinct from patients with CVST before the COVID-19 pandemic, with high rates of coma and intracerebral hemorrage and a high mortality rate.
28 September
Siegler AJ, Luisi N, Hall EW, et al. Trajectory of COVID-19 Vaccine Hesitancy Over Time and Association of Initial Vaccine Hesitancy With Subsequent Vaccination. JAMA Netw Open September 24, 2021;4(9):e2126882. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2784480
This large cohort study found that COVID-19 vaccine hesitancy is not a stable trait. More than one-third (37%) transitioned from vaccine hesitant into vaccine willing within a few months (by normal variables: sex, age, race, age, etc).
26 September
El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. NEJM September 22, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2113017?query=featured_home
Longer follow up of this large RCT in 30,415 participants (interim analysis has been published on December 30, 2020 https://www.nejm.org/doi/full/10.1056/NEJMoa2035389). At a median follow-up of 5.3 months in the blinded phase, the efficacy of the MODERNA vaccine in preventing severe disease was 98.2%, with 2 versus 106 cases in the placebo group. Vaccine efficacy was consistent across ethnic and racial groups, age groups, and participants with coexisting conditions.
Pilishvili T, Gierke R, Fleming-Dutra KE, et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. NEJM September 22, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2106599?query=featured_home
Among 1482 case and 3449 control participants, vaccine effectiveness for complete vaccination was 88.8% (95% CI, 84.6 to 91.8) with the BNT162b2 vaccine (Pfizer–BioNTech) and 96.3% (95% CI, 91.3 to 98.4) with the mRNA-1273 vaccine (MODERNA).
Haas EJ, McLaughlin JM, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Inf Dis September 22, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00566-1/fulltext
Without the national vaccination campaign, Israel probably would have had triple the number of hospitalisations and deaths compared with what actually occurred during its largest wave of the pandemic to date, and the health-care system might have become overwhelmed.
Wagner CE, Saad-Roy CM, Morris SE, et al. Vaccine nationalism and the dynamics and control of SARS-CoV-2. Science September 24, 2021, Vol. 373, NO. 656224. https://www.science.org/doi/10.1126/science.abj7364
Complex modeling study showing that unequal vaccine allocation will result in sustained transmission and increased case numbers in regions with low vaccine availability and thus to a higher associated clinical burden compared with a vaccinated population. Moreover, under certain scenarios, sustained local transmission could lead to an increased potential for antigenic evolution.
Nunes B, Rodrigues AP, Kislaya I, et al. mRNA vaccine effectiveness against COVID-19-related hospitalisations and deaths in older adults: a cohort study based on data linkage of national health registries in Portugal, February to August 2021. Euro Surveill. 2021;26(38):pii=2100833. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.38.2100833
High mRNA vaccine effectiveness in older people in Portugal, supporting data from Israel and U.S. Of note, there was no evidence of VE reduction up to 3 months after the second dose and during the period of Delta variant circulation.
Zhu F, Jin P, Zhu T, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomised, double-blind, placebo-controlled, phase 2b trial. Clinical Infectious Diseases September 22, 2021, ciab845, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab845/6374123
An Ad5-vectored COVID-19 vaccine (from China) with a single dose was safe and induced robust immune responses in 150 children and adolescents aged 6-17 years.
24 September
Dooling K, Gargano JW, Moulia D, et al. Use of Pfizer-BioNTech COVID-19 Vaccine in Persons Aged ≥16 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, September 2021. MMWR Morb Mortal Wkly Rep 21 September 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7038e2.htm?s_cid=mm7038e2_w
After 8 months of use under an FDA EUA and CDC ACIP interim recommendation, the Pfizer-BioNTech COVID-19 vaccine, Comirnaty, now has full FDA approval and is recommended by ACIP for use in persons aged ≥ 16 years in the US. A brief discussion.
Paulsen FO, Schaefers C, Langer F, et al. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19). Blood September 16, 2021, 138 (11): 996–999. https://ashpublications.org/blood/article/138/11/996/476455/Immune-thrombocytopenic-purpura-after-vaccination
Four patients with symptomatic thrombocytopenia associated with previous administration of ChAdOx1 nCov-19 (but not associated with VITT). Of note, 3 of 4 patients presented with a medical history known to be associated with the occurrence of thrombocytopenia.
23 September
Sokal A, Barba-Spaeth G, Fernández I, et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity September 20, 2021. https://www.cell.com/immunity/fulltext/S1074-7613(21)00396-4
Although naïve individuals had weaker neutralizing serum responses than recovered patients, many of their RBD-specific memory B cells (MBCs) displayed high affinity towards multiple variants of concern (VOCs), including Delta and Beta. These data suggest that an additional challenge in naive vaccinees could recall such affinity-matured MBCs and allow them to respond efficiently to VOCs.
21 September
Self WH, Tenforde MW, Rhoads JP, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep 17 September 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7038e1.htm?s_cid=mm7038e1_w
Vaccine effectiveness against COVID-19 hospitalization during March 11–August 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%). However, as with all real-world data, residual confounding is possible.
Warren CM, Snow TT, Lee AS, et al. Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines With Confirmatory Testing in a US Regional Health System. JAMA Netw Open September 17, 2021;4(9):e2125524. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2784268?resultClick=1
Immunological testing of 22 patients with suspected vaccine allergy suggests that non–IgE-mediated allergic reactions to polyethylene glycol may be responsible for many cases of allergy to mRNA vaccines.
20 September
Falsey AR, Frenck RW, Walsh EE, et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. NEJM September 15, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2113468?query=featured_home
Preliminary findings from a small pivotal trial, suggesting that a third dose could prolong protection and further increase the breadth of protection.
18 September
Today we’ll have a Vaccines special. A lot of data on the long-term efficacy of the BioNTech vaccine and some arguments for a third jab in older people. Some more data on breakthrough infections, waning immunity, on immune correlates of protection and on how to identify VITT cases.
Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. NEJM September 15, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2114255?query=featured_home
Paper of the day, providing important data from the Israeli Ministry of Health on a third shot (“booster”). Among 1,137,804 persons who were 60 years of age or older and had been fully vaccinated (two doses of BNT162b2) at least 5 months earlier, the rate of confirmed infection was lower in the booster group by a factor of 11.3; the rate of severe illness was lower by a factor of 19.5. Though sources of bias may not have been measured or corrected for adequately, these data strongly argue for a third shot in the elderly.
Thomas SJ, Moreira ED, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. NEJM September 15, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2110345?query=featured_home
Follow up of the large Phase III RCT (published a few months ago), showing a gradual decline in vaccine efficacy over time. Efficacy peaked at 96.2% during the interval from 7 days to less than 2 months after the second dose and declined gradually to 83.7% from 4 months after the second dose to the data cutoff date — an average decline of approximately 6% every 2 months.
Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep September 15, 2021;70:1306–1311. https://www.cdc.gov/mmwr/volumes/70/wr/mm7037a7.htm?s_cid=mm7037a7_w
Same direction in the “real world”. From May 3–July 25, 2021, the overall age-adjusted vaccine effectiveness against hospitalization in New York was relatively stable. However, the overall age-adjusted vaccine effectiveness against infection for all New York adults declined from 91.8% to 75.0%, coinciding with a period of easing societal public health restrictions and increasing Delta variant circulation.
Salih F, Schönborn L, Kohler S, et al. Vaccine-Induced Thrombocytopenia with Severe Headache. NEJM September 15, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2112974?query=featured_home
Small case series, providing some evidence that vaccine-induced thrombocytopenia (VIT) without associated cerebral venous sinus thrombosis and with severe headache as the heraldic symptom may precede VITT (“pre-VITT syndrome”). The authors argue that patients who present with severe headache 5 to 20 days after adenovirus vector vaccination should undergo immediate testing for thrombocytopenia and d-dimer levels.
Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science September 17, 2021. Vol 373, Issue 6561. https://www.science.org/doi/10.1126/science.abj0299
In monkeys, S-specific binding antibody induced by the Moderna vaccine was a surrogate marker of protection. Moreover, protection of the lower respiratory tract required lower serum antibody concentrations, possibly explaining why most current vaccines are highly effective against severe lower airway disease.
Xia L, Zhang YT, Wang YX, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Inf Dis September 15, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00462-X/fulltext
The inactivated COVID-19 vaccine BBIBP-CorV was safe and well-tolerated at all tested dosing levels in 288 participants aged 3–17 years. The vaccine also elicited robust humoral responses.
16 September
Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med September 15, 2021. https://www.nature.com/articles/s41591-021-01527-y
This very preliminary evaluation described the antibody persistence of mRNA-1273 and the safety and immunogenicity of a booster dose of mRNA-1273 and two updated versions (mRNA-1273.351 and mRNA-1273.211) in 80 participants who had been vaccinated 6 months previously with the authorized schedule of mRNA-1273. The bottom line: tolerability was good (“generally similar to (that) observed after the primary series”). More importantly: booster vaccination with all 3 vaccines (seemingly, no big differences between them) induced strong anamnestic responses, indicative of a robust B cell memory response.
Van Praet JT, Vandecasteele S, De Roo A, et al. Dynamics of the cellular and humoral immune response after BNT162b2 mRNA Covid-19 vaccination in Covid-19 naive nursing home residents. J Infect Dis September 13, 2021; jiab458, https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiab458/6369254
Among nursing home residents after BNT162b2 mRNA COVID-19 vaccination, both humoral and cellular responses significantly decreased over the course of 24 weeks and were substantially lower than those of healthcare workers at all time points. The half-life of the antibody response was only 47 days, indicating a quantitatively lower immune reaction and shorter duration of protection for the residents.
15 September
Schönborn L, Thiele T, Kaderali L, et al. Decline in Pathogenic Antibodies over Time in VITT. NEJM September 8, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2112760?query=featured_home
Anti-PF4 antibodies are transient in most patients with VITT. In 14/15 patients with follow-up of more than 12 weeks, the platelet-activation assay became negative.
14 September
Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. NEJM September 8, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2110362?query=featured_home
Real-world data from the US. The effectiveness of mRNA-based vaccines was 88% against a SARS-CoV-2 infection leading to hospitalization and 90% against infection leading to ICU admission. This was also seen in those most at risk for severe COVID-19 (advanced age, underlying medical conditions, race or ethnic group). However, it is of note that during this observation period, the Alpha variant was predominant.
Grannis SJ, Rowley EA, Ong TC, et al. Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19–Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance — Nine States, June–August 2021. MMWR Morb Mortal Wkly Rep 10 September 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7037e2.htm?s_cid=mm7037e2_w
What about Delta? Here, the same network as above provided data from 187 hospitals and 221 emergency departments (EDs) and urgent care (UC) clinics across nine US states June–August 2021. Vaccine effectiveness (VE) of all three US-authorized COVID-19 vaccines combined remained high against hospitalization (86%) and ED/UC encounters (82%). However, VE among adults aged ≥ 75 years was significantly lower. This decline should be interpreted with caution and might be related to changes in SARS-CoV-2, waning of vaccine-induced immunity, or a combination of factors.
Bajema KL, Dahl RM, Prill MM, et al. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19–Associated Hospitalization — Five Veterans Affairs Medical Centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly Rep 10 September 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7037e3.htm?s_cid=mm7037e3_w
Same direction: mRNA COVID-19 vaccines remain highly effective, even during periods of widespread circulation of the Delta variant. However, VE in preventing COVID-19–related hospitalization was 80% among adults aged ≥ 65 years compared with 95% among adults aged 18–64 years. Growing evidence that the vaccines start to weaken when Delta catches the elderly.
13 September
Sauré D, O’Ryan M, Torres JP, et al. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Inf Dis September 09, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00479-5/fulltext
In this huge study, IgG seropositivity was lower after CoronaVac than after BNT162b2 and declined over time since vaccination for CoronaVac recipients but not BNT162b2 recipients.
12 September
To KK, Li Y, Lung DC et al. False COVID-19 cases due to contamination by inactivated virus vaccine. Clin Inf Dis September 9, 2021, ciab684, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab684/6367446
Two individuals whose respiratory specimens were contaminated by inactivated SARS-CoV-2 vaccine strain (CoronaVac), likely at vaccination premises.
8 September
Klein NP, Lewis N, Goddard K, et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA September 3, 2021. https://jamanetwork.com/journals/jama/fullarticle/2784015
They. Are. Safe. In this interim analysis of surveillance monitoring of more than 11.8 million doses of 2 mRNA vaccines in diverse populations and weekly analyses from December 14, 2020, to June 26, 2021, no vaccine-outcome association met the prespecified threshold for a signal.
Seppälä E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill September 2, 2021;26(35):pii=2100793. https://doi.org/10.2807/1560-7917.ES.2021.26.35.2100793
The adjusted VE against infection with the Delta variant was 22% among those partly vaccinated and 65% among those fully vaccinated, compared with 55% and 84%, respectively, against the Alpha variant.
6 September
Klein NP, Lewis N, Goddard K. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA September 3, 2021. https://jamanetwork.com/journals/jama/fullarticle/2784015?resultClick=1
In this interim analysis of 6.2 million persons who received 11.8 million doses of an mRNA vaccine, event rates for 23 serious health outcomes were not significantly higher for individuals 1 to 21 days after vaccination compared with similar individuals at 22 to 42 days after vaccination.
Feder KA, Patel A, Vepachedu VR, et al. Association of E484K spike protein mutation with SARS-CoV-2 infection in vaccinated persons—Maryland, January – May 2021, Clin Inf Dis September 2, 2021, ciab762, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab762/6362726
Among 9048 cases, SARS-CoV-2 viruses carrying the spike protein mutation E484K were disproportionately prevalent among persons infected after full vaccination (adjusted OR 1.96).
5 September
Ben David SSS, Potasman I, Rahamim-Cohen D. Rate of Recurrent Guillain-Barré Syndrome After mRNA COVID-19 Vaccine BNT162b2. JAMA Neurol September 1, 2021; https://jamanetwork.com/journals/jamaneurology/fullarticle/2783708?resultClick=1
In this cohort study of 702 patients, only 1 person needed short medical care for relapse of their previous syndrome, representing a minimal risk.
Marlin R, Godot V, Cardinaud S, et al. Targeting SARS-CoV-2 receptor-binding domain to cells expressing CD40 improves protection to infection in convalescent macaques. Nat Commun September 1, 2021, 12, 5215. https://www.nature.com/articles/s41467-021-25382-0
A new generation of subunit vaccines targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike antigen to the CD40 receptor (αCD40.RBD). Immunogenicity is shown in two different animal models. This vaccine may have advantages for a safe and efficient boosting strategy, without requiring an adjuvant.
4 September
Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet September 01, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01699-8/fulltext
Sub-study of two trials in which the timing of the second dose varied and allowed for comparisons of immunogenicity between the recommended vaccination schedule and a longer interval. Results: the extended interval between the first two doses (44–45 weeks) resulted in higher antibody titers after the second dose than with a shortened interval. A third dose given 28–38 weeks after the second dose increased the antibody titers to above those after the primary (1-2) series.
McQuade ET, Breskin A. Longer intervals and extra doses of ChAdOx1 nCoV-19 vaccine. Lancet September 01, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01817-1/fulltext
But what do these data tell us? In their comment, Elizabeth Rogawski McQuade and Alexander Breskin point out that “the total public health impact of the extended prime-boost interval is unclear given the trade-off between a longer period at the lower level of protection afforded by a single dose and the higher level of protection obtained after a delayed second dose”. However, data may assuage concerns about the potential for impaired responses after repeated use of a replication deficient simian adenoviral vector and suggest that a third dose of the AZ/Oxford vaccine could be successful if necessary.
2 September
Shavit R, Maoz-Segal R, Iancovici-Kidon M, et al. Prevalence of Allergic Reactions After Pfizer-BioNTech COVID-19 Vaccination Among Adults With High Allergy Risk. JAMA Netw Open August 31, 2021; 4(8):e2122255. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2783626?resultClick=1
In this cohort study of 8102 individuals with a history of allergies, an algorithm was used to define 429 (5%) as “highly allergic”; this group was referred to receive immunization under medical supervision. A total of 98% of the highly allergic individuals had no allergic reaction, 6 (1%) had mild allergic responses, and 3 (0.7%) had anaphylactic reactions.
Deepak P, Kim W, Paley MA. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2. Annals Int Med August 31, 2021. https://www.acpjournals.org/doi/10.7326/M21-1757
This study shows that compared with non-users, patients with chronic inflammatory disease treated with glucocorticoids and B cell depletion therapy seem to have lower SARS-CoV-2 vaccine-induced antibody responses.
1 September
Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA August 30, 2021. https://jamanetwork.com/journals/jama/fullarticle/2783797?resultClick=1
This large prospective cohort study in Belgian HCWs demonstrated a significantly higher humoral immunogenicity of the mRNA-1273 vaccine (Moderna) compared with the BNT162b2 vaccine (Pfizer-BioNTech), in infected as well as in uninfected participants, and across age categories. According to the authors, the higher mRNA content in mRNA-1273 compared with BNT162b2 and the longer interval between priming (4 weeks vs 3 weeks for BNT162b2) might explain this difference.
Knowlton KU. Insights from a murine model of COVID-19 mRNA vaccination-induced myopericarditis: Could accidental intravenous vaccine injection induce myopericarditis? Clin Inf Dis August 28, 2021, ciab741 https://doi.org/10.1093/cid/ciab741
Kirk Knowlton from Salt Lake City believes that the current data suggest that this is plausible and that it would be appropriate to consider further.
30 August
Bates TA, Leier HC, Lyski ZL, et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun August 26, 2021, 12, 5135. https://doi.org/10.1038/s41467-021-25479-6
This study indicates that B.1.1.7 (Alpha) and B.1.351 (Beta) are less well neutralized by serum from vaccinated individuals, and that B.1.351, but not B.1.1.7, is less well neutralized by convalescent serum.
29 August
Uzun G, Althaus K, Bakchoul T. No Correlation between Anti-PF4 and Anti–SARS-CoV-2 Antibodies after ChAdOx1 nCoV-19 Vaccination. NEJM August 25, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2111305?query=featured_home
This study does not support the hypothesis that the immune response against SARS-CoV-2 proteins leads to the formation of anti-platelet factor 4 antibodies in patients with vaccine-induced immune thrombotic thrombocytopenia.
28 August
Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. NEJM August 25, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2110475?query=featured_home
This observational data set involving more than 2.4 million vaccinated persons from Israel identified an excess risk of lymphadenopathy (78.4 events per 100,000 persons), herpes zoster infection (15.8 events), appendicitis (5.0 events), and myocarditis (2.7 events). Compared to the risk of adverse events associated with SARS-CoV-2 infection, this is almost meaningless.
Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ August 27, 2021; 374 doi: https://doi.org/10.1136/bmj.n1931
Same direction as above. This patient level data obtained for approximately 30 million people vaccinated in England shows that the slightly increased risks of severe hematological and vascular events after the first doses of the Oxford/AZ and Pfizer/BNT vaccines were minimal vs the substantially higher and more prolonged events after SARS-CoV-2 infection in the same population.
27 August
Baltas I, Boshier FA, Williams CA, et al. Post-vaccination COVID-19: A case-control study and genomic analysis of 119 breakthrough infections in partially vaccinated individuals. Clinical Infectious Diseases, August 19, 2021, ciab714, https://doi.org/10.1093/cid/ciab714
This matched control study from the UK describes a cohort of Pfizer/BNT or AZ/Oxford vaccinated multimorbid patients developing COVID-19 predominantly from the B.1.1.7 lineage post first vaccination. One life was saved every four to five vaccinations. As mortality benefit from vaccination occurred immediately after COVID-19 infection, these data indirectly question whether pauci-symptomatic/asymptomatic patients should be offered vaccination.
26 August
Today we have a special on vaccine effectiveness towards the B.1.617.2 (Delta).
Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. ePub: 24 August 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034e4.htm?s_cid=mm7034e4_x
In this cohort of 4217 frontline workers (mainly HCW), the vaccine effectiveness (VE) declined from 91% to 66% when the SARS-CoV-2 Delta variant became predominant. However, this trend should be interpreted with caution (increasing time since vaccination, low numbers).
Behrens GM, Cossmann A, Stankov MV, et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet August 17, 2021. https://doi.org/10.1016/S0140-6736(21)01891-2
Robust inhibition of variants including Delta by the switch vaccination of AZ then Pfizer/BNT.
Hammerschmidt SI, Bosnjak B, Bernhardt G, et al. Neutralization of the SARS-CoV-2 Delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Cell Mol Immunol August 23, 2021. https://doi.org/10.1038/s41423-021-00755-z
Same group, same direction. This small study indicates an overall robust inhibition of the Delta variant by heterologous boosting with Pfizer/BNT of vaccinees initially primed with AZ. However, in contrast to Alpha, Beta, and Gamma variants, homologous Pfizer/BNT prime-boost vaccination appeared to be even more efficient in neutralizing the Delta variant.
Griffin JB, Haddix M, Danza P, et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status — Los Angeles County, California, May 1–July 25, 2021. MMWR Morb Mortal Wkly Rep. ePub: 24 August 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034e5.htm?s_cid=mm7034e5_w
From May 1–July 25, 2021, among 43,127 SARS-CoV-2 infections in residents of Los Angeles County, 25.3% were in fully vaccinated persons, 3.3% were in partially vaccinated persons, and 71.4% were in unvaccinated persons. On July 25, infection and hospitalization rates among unvaccinated persons were 4.9 and 29.2 times, respectively, those of fully vaccinated persons. Of note, in July, when Delta was predominant, cycle threshold values were similar for unvaccinated and vaccinated persons.
Ong SW, Chiew CJ, Ang LW, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Inf Dis August 23, 2021; ciab721, https://doi.org/10.1093/cid/ciab721
Retrospective cohort from Singapore, indicating a signal toward increased severity associated with B.1.617.2 (Delta). The association of B.1.617.2 with lower cycle threshold value and longer viral shedding provides a potential mechanism for increased transmissibility. All of 18 vaccinated patients with Delta had mild disease and none developed pneumonia.
25 August
Ranzani OT, Hitchings MD, Durion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ August 20, 2021; 374. doi: https://doi.org/10.1136/bmj.n2015
Moderate results with the inactivated whole virus vaccine CoronaVac (from Sinovac Biotech) in older people (> 70 years) in a setting with extensive transmission of the gamma variant: adjusted vaccine effectiveness against symptomatic COVID-19 was 24.7% at 0-13 days and 46.8% at ≥ 14 days after the second dose. Adjusted vaccine effectiveness against hospital admissions was 55.5% (death 61.2%) at ≥ 14 days after the second dose, declining with increasing age.
Wan EY, Chui CD, Lai FT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Inf Dis August 16, 2021. DOI: https://doi.org/10.1016/S1473-3099(21)00451-5
An additional 4.8 cases of this generally self-limiting adverse event per 100,000 people vaccinated with CoronaVac and 2.0 cases per 100,000 people vaccinated with BNT162b2.
24 August
Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ August 20, 2021; 374. doi: https://doi.org/10.1136/bmj.n1943
Two doses of mRNA COVID-19 vaccines were observed to be highly effective against symptomatic infection and severe outcomes. Vaccine effectiveness of one dose was observed to be lower, particularly for older adults shortly after the first dose. Of note, a higher effectiveness was seen after one dose of mRNA-1273 (MODERNA) than after one dose of BNT162b2 (BioNTech/Pfizer).
Debes AK, Yiao S, Colantuoni E, et al. Association of Vaccine Type and Prior SARS-CoV-2 Infection With Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers. JAMA Intern Med August 16, 2021; https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2782821
You don’t have to suffer to benefit from COVID vaccination. In this study on HCWs, the vast majority of participants (953 of 954!) developed spike IgG antibodies 14 or more days following dose 2, regardless of vaccine reactions!
23 August
Eliakim-Raz N, Massarweh A, Stemmer A, Stemmer SM. Durability of Response to SARS-CoV-2 BNT162b2 Vaccination in Patients on Active Anticancer Treatment. JAMA Oncol. 2021 Aug 11:e214390. PubMed: https://pubmed.gov/34379092. Full text: https://doi.org/10.1001/jamaoncol.2021.4390
Anti-spike (anti-S) IgG antibody response to the BioNTech/Pfizer vaccine in 95 patients with solid tumors on active anti-cancer treatment after a median of 4 months from the second vaccination. Eighty-three patients (87%) were seropositive for anti-S IgG antibodies. The median titer levels in patients with cancer was significantly lower than those in the control group. There was a 3.6-fold range in median titer values across tumor types and a wider range (8.8-fold) across treatment types. The only variable significantly associated with lower IgG titers was treatment with chemotherapy plus immunotherapy and immunotherapy plus biological therapy.
22 August
Gilbert PB, Montefiori DC, McDermott A, et al. Immune Correlates Analysis of the mRNA-1273 COVID-19 Vaccine Efficacy Trial. medRxiv 2021, posted 10 August. Full text: https://doi.org/10.1101/2021.08.09.21261290
Antibody levels (measured as IgG bAbs to spike, IgG bAbs to spike RBD, ID50 and ID80 nAb titer) might be able to predict the level of protection provided by the Moderna vaccine. This is the result of a study which compared levels of neutralizing antibodies in 47 vaccinated individuals who developed breakthrough infections with matched controls.
21 August
Morris J. Israeli data: How can efficacy vs. severe disease be strong when 60% of hospitalized are vaccinated? Covid-19 Data Science 2021, posted 17 August. Full text: https://www.covid-datascience.com/post/israeli-data-how-can-efficacy-vs-severe-disease-be-strong-when-60-of-hospitalized-are-vaccinated
“It is important to use infection and disease rates (per 100k, e.g.) and not raw counts to compare unvaccinated and vaccinated groups to adjust for the proportion vaccinated. Use of raw counts exaggerates the vaccine efficacy when vaccinated proportion is low and attenuates the vaccine efficacy when, like in Israel, vaccines proportions are high.”
Copyright: Jeffrey Morris
Ollila TA, Lu S, Masel R, et al. Antibody Response to COVID-19 Vaccination in Adults With Hematologic Malignant Disease. JAMA Oncol. 2021 Aug 11:e214381. PubMed: https://pubmed.gov/34379085. Full text: https://doi.org/10.1001/jamaoncol.2021.4381
Retrospective study of 160 adults with hematologic malignant disease who were vaccinated with a COVID-19 vaccine. (One hundred and five (66%) patients received a B cell–depleting monoclonal antibody, most commonly rituximab (n = 85)). Sixty-three patients (39%) demonstrated seroconversion. Longer time (greater than or less than 12 months) from last chemotherapy administration to vaccination was associated with increased rates of seroconversion. The quantitative antibody response was also lower among patients with exposure to B cell/plasma cell–depleting antibodies and those with active malignant disease.
20 August
Tan CW, Chia WN, Young BE, et al. Pan-Sarbecovirus Neutralizing Antibodies in BNT162b2-Immunized SARS-CoV-1 Survivors. N Engl J Med. 2021 Aug 18. PubMed: https://pubmed.gov/34407341. Full text: https://doi.org/10.1056/NEJMoa2108453
If you had survived SARS-CoV-1 in 2002-2004, the BioNTech/Pfizer vaccine would today give you potent cross-clade pan-sarbecovirus neutralizing antibodies. This study (n = 8) is another proof of concept that a pan-coronavirus vaccine is possible. Such a vaccine might cover not only SARS-CoV-2 and its current and future variants but also other coronaviruses with known potential to cause severe human diseases.
Mallapaty S. Delta’s rise is fuelled by rampant spread from people who feel fine. Nature 2021, published 19 August. Full text: https://www.nature.com/articles/d41586-021-02259-2
“People infected with the Delta variant generally do not have COVID-19 symptoms until two days after they start shedding the coronavirus.”
19 August
Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. ePub: 18 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7034e3
“Two doses of mRNA vaccines were 74.7% effective against infection among nursing home residents early in the vaccination program (March–May 2021). During June–July 2021, when B.1.617.2 (Delta) variant circulation predominated, effectiveness declined significantly to 53.1%.”
Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet 2021, published 17 August. Full text: https://doi.org/10.1016/S2352-3018(21)00157-0
A double-blind, placebo-controlled, Phase 1B/2A study of the AstraZeneca vaccine in 104 people with HIV and 70 HIV-negative controls. The authors found similar full-length spike (FLS)-binding and receptor-binding domain (RBD)-binding IgG and SARS-CoV-2 neutralizing response patterns in people with HIV and HIV-negative SARS-CoV-2-naive participants.
Schmitz AJ, Turner JS, Liu Z, et al. A vaccine-induced public antibody protects against SARS-CoV-2 and emerging variants. Immunity 2021, published 16 August. Full text: https://doi.org/10.1016/j.immuni.2021.08.013
The authors describe an antibody, dubbed 2C08 (a SARS-CoV-2 vaccine-induced mAb cloned from a germinal center B cell isolated from a draining axillary lymph node sampled from a healthy adult after receiving their second dose of an mRNA-based vaccine) which potently neutralizes the Delta, Gamma and Alpha strains and reduces lung viral load and morbidity in hamsters challenged with Delta and Gamma. Clonal analysis identified 2C08-like public clonotypes among B cells responding to SARS-CoV-2 infection or vaccination in 41 out of 181 individuals. Ergo: SARS-CoV-2 vaccines mitigate resistance by circulating variants of concern. Tell your friends to get vaccinated.
18 August
Wadman M. A grim warning from Israel: Vaccination blunts, but does not defeat Delta. Science 2021, published 16 August. Full text: https://www.sciencemag.org/news/2021/08/grim-warning-israel-vaccination-blunts-does-not-defeat-delta
“Israel, which has led the world in launching vaccinations and in data gathering, is confronting a surge of COVID-19 cases that officials expect to push hospitals to the brink. Nearly 60% of gravely ill patients are fully vaccinated.”
Lloyd-Sherlock P, Lasco G, McKee M, Perianayagam A, Sempé L. Does vaccine ageism amount to gerontocide? Lancet. 2021 Aug 11:S0140-6736(21)01689-5. PubMed: https://pubmed.gov/34390657. Full text: https://doi.org/10.1016/S0140-6736(21)01689-5
“In India, more people younger than 45 years are being vaccinated than those 60 years or older, even though about half of those 60 years or older are yet to receive even a single dose. Unlike age-based triage for acute COVID-19 care, this vaccination policy will not save lives: it will contribute to thousands, potentially millions of avertable deaths. In the Philippines, where only 8·5% of people 60 years or older had been fully vaccinated as of June 29, 2021, the focus of vaccination has now shifted to younger so-called working age adults.”
17 August
Evans SJW, Jewell NP. Vaccine Effectiveness Studies in the Field. N Engl J Med. 2021 Jul 12. PubMed: https://pubmed.gov/34289274. Full-text: https://doi.org/10.1056/NEJMoa2108891.
“Questions crucial to vaccination policy (…) include the effect of new virus variants, the timing between vaccine doses, the effect of vaccines on asymptomatic infection in contrast to severe disease, the waning of vaccine immunity, and the potentially enhanced effectiveness of mix-and-match strategies that might be used with booster shots.” A comment on the paper we presented on 23 July: Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021 Aug 12;385(7):585-594. PubMed: https://pubmed.gov/34289274. Full text: https://doi.org/10.1056/NEJMoa2108891
Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021 Aug 4. PubMed: https://pubmed.gov/34347001. Full text: https://doi.org/10.1001/jama.2021.13443
Among 2,000,287 individuals receiving at least 1 COVID-19 vaccination (BioNTech/Pfizer: 52.6%, Moderna: 44.1%, Johnson & Johnson: 3.1%), 20 individuals had vaccine-related myocarditis (1 per 100,000) and 37 had pericarditis (1.8 per 100,000). Myocarditis occurred a median of 3.5 days after vaccination. Fifteen individuals (75%) were male. Four persons (20%) developed symptoms after the first vaccination and 16 (80%) after the second one. Nineteen patients (95%) were admitted to the hospital. All were discharged after a median of 2 days. There were no readmissions or deaths. Find more details about pericarditis in the article.
FDA 20210812. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA 2021, published 12 August. Full text: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised
The FDA allows a third booster dose in individuals with solid organ transplant or those who have an equivalent level of immunocompromise. The news release also states that “patients should be counseled to maintain physical precautions to help prevent COVID-19. In addition, close contacts of immunocompromised persons should get vaccinated, as appropriate for their health status, to provide increased protection to their loved ones.”
16 August
Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Dis 2021, published 12 August. Full text: https://doi.org/10.1016/S2213-2600(21)00357-X
A new prospective vaccine mixing study comparing “AstraZeneca (AZ) + BioNTech/Pfizer (BP)” with 2 x AZ and 2 x BP. The authors show “AZ first, BP second” elicited a stronger immune response than two doses of either vaccine. Could these data spur a renaissance for the AstraZeneca vaccine? Not sure. The difference might be explained by the longer (and possibly more effective) immunization interval of AZ+BP compared to the typical 3-week interval of the two BP injections.
Hou X, Zaks T, Langer R, et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater (2021). Full text: https://doi.org/10.1038/s41578-021-00358-0
The authors discuss the physiological barriers and possible administration routes for lipid nanoparticle–mRNA systems and highlight preclinical and clinical studies of lipid nanoparticle–mRNA therapeutics for infectious diseases, cancer and genetic disorders.
15 August
Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021 Aug 11. PubMed: https://pubmed.gov/34379917. Full text: https://doi.org/10.1056/NEJMc2111462
A double-blind, randomized, controlled trial of a third-dose booster of the Moderna vaccine in 120 organ-transplant recipients (median time from transplantation to the third dose was 3.16 years). After four months, an anti–receptor-binding domain (RBD) antibody level of at least 100 U per milliliter was present in 33 of 60 patients (55%) in the mRNA-1273 group and in 10 of 57 patients (18%) in the placebo group. The median percent virus neutralization was 71% in the Moderna group and 13% in the placebo group. The trial was not powered to detect differences in clinical outcomes.
14 August
Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med. 2021 Aug 11. PubMed: https://pubmed.gov/34379915. Full text: https://doi.org/10.1056/NEJMoa2109522
Nothing truly new: the Moderna vaccine had an acceptable safety profile in adolescents, the immune response was similar to that in young adults, and the vaccine prevented COVID-19. Interestingly, in the placebo group, after the first and second injections, study participants experienced injection-site pain (in 34.8% and 30.3%, respectively), headache (in 38.5% and 30.2%, respectively), and fatigue (in 36.6% and 28.9%, respectively). It’s fascinating how just the thought of getting a vaccine that might give you headache or fatigue is sufficient to give you… headache fatigue. In this trial, about half of all mild adverse events were probably the product of human imagination.
Pegu A, O’Connell S, Schmidt SD, et al. Durability of mRNA-1273-induced antibodies against SARS-CoV-2 variants. Science 2021, published 12 PubMed: https://pubmed.gov/34031659. Full text: https://doi.org/10.1126/science.abj4176
Most individuals vaccinated with the Moderna vaccine maintained binding and functional antibodies against SARS-CoV-2 variants for 6 months, including Alpha, Beta, Gamma, B.1.429, and B.1.526. Neutralizing responses were rare after a single Moderna dose, but at the peak of response to the second dose, all individuals had robust responses to all variants. The study included the results from 8 volunteers in each of three age groups: 18-55, 55-70, and 71+ years of age.
Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 11, 142 (2021). https://doi.org/10.1038/s41408-021-00534-z
In patients with hematological malignancies, vaccination with two doses of the BioNTech/Pfizer vaccine translates into a significant increase in humoral response, allowing almost half of the patients to achieve immune protection against COVID-19 (retrospective study, n = 237). The use of B cell targeting treatment within the previous 12 months before vaccination, and a low CD19+ B cell level predicted failure in achieving immune protection.
Baraniuk C. Covid-19: How effective are vaccines against the delta variant? BMJ. 2021 Aug 9;374:n1960. PubMed: https://pubmed.gov/34373255. Full text: https://doi.org/10.1136/bmj.n1960
49% of people in the UK who died from infection with the SARS-CoV-2 Delta variant up until 19 July had had two vaccine doses – the author is confident about the protection offered by current vaccines. (He’s probably right!)
13 August
The bad news: Immunity wanes over time
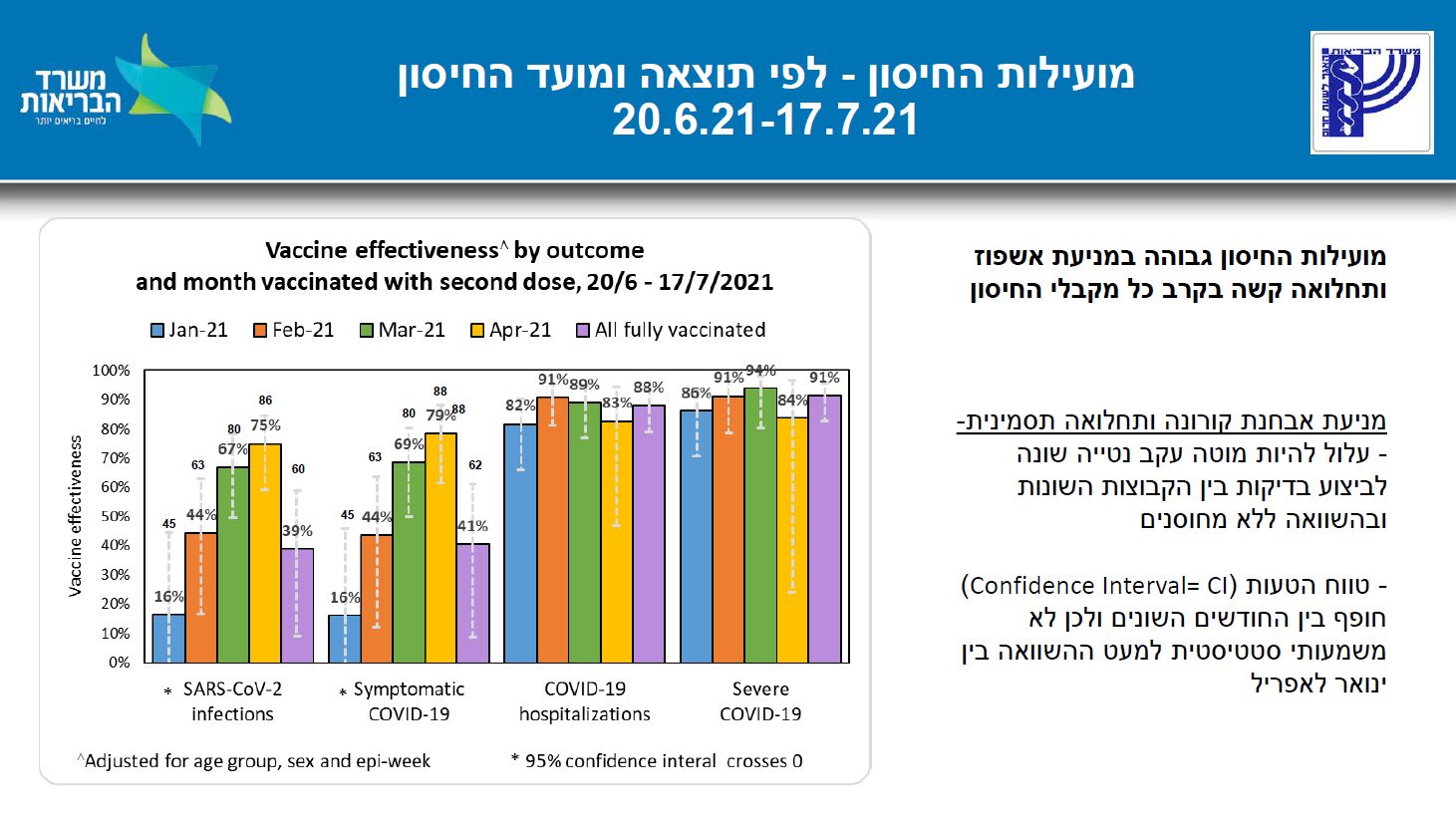
IMH 20210812. Concentration of data on vaccinated in two doses until 31/1/2021 (discussion from 20.7.2021) [ריכוז נתונים על מחוסנים בשתי מנות עד לתאריך 31/1/2021(דיון מ 20.7.2021)]. Israel Ministry of Health (משרד הבריאות) 2021, last modified 12 August. PDF with slides: https://www.gov.il/BlobFolder/reports/vaccine-efficacy-safety-follow-up-committee/he/files_publications_corona_two-dose-vaccination-data.pdf
Effectiveness of the BioNTech/Pfizer vaccine in Israel by 1) outcome and 2) month vaccinated with second dose (slide 8):
The good news: Waning immunity is better than no immunity
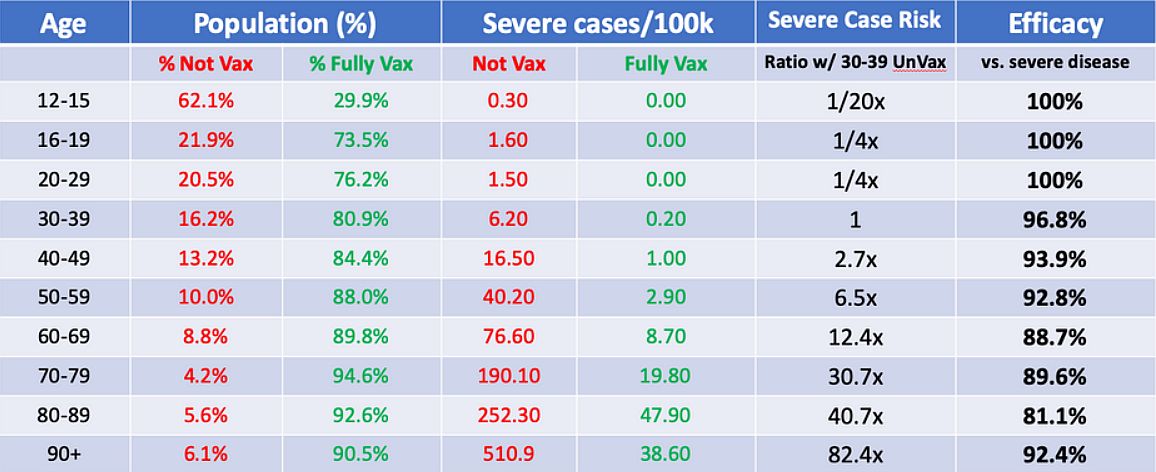
Jeffey N. Among older Israelis, serious COVID rate six times as high if unvaccinated. The Times of Israel 2021, published 10 August. Full text: https://www.timesofisrael.com/among-older-israelis-serious-covid-rate-six-times-higher-if-unvaccinated/
In the new epidemic wave in Israel, the number of severe COVID-19 cases is far higher among vaccinated people, both old and young.
Van Vinh Chau N, Ngoc NM. Transmission of SARS-CoV-2 Delta Variant Among Vaccinated Healthcare Workers, Vietnam. Lancet Preprints 2021, posted 10 August.
In this study of 62 healthcare workers, breakthrough infections with the Delta variant were associated with high viral loads (251 times higher than in people infected with historical strains), prolonged PCR positivity (8–33 days; median: 21), and low levels of vaccine-induced neutralizing antibodies. The authors conclude that physical distancing measures will be critical to reduce the transmission of the Delta variant.
12 August
Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021, posted 9 August. Full text: https://doi.org/10.1101/2021.08.06.21261707
Data from the Mayo Clinic Health System from January to July 2021 find that the mRNA vaccines produced by BioNTech/Pfizer and Moderna are highly effective against SARS-CoV-2 infection (BioNTech/Pfizer: 76%; Moderna: 86%) and COVID-19 associated hospitalization (85% vs. 91.6%). Surprise in July: while vaccine effectiveness against hospitalization remained high (75% vs 81%), effectiveness against infection was lower for both vaccines (42% vs 76%), with a more pronounced reduction for the BioNTech/Pfizer vaccine. To be taken with a grain of salt. (BioNTech/Pfizer vaccine administered earlier than the Moderna vaccine?) Note that this is a pre-print paper that has not yet been reviewed.
Hadjadj J, Planas D, Ouedrani A, et al. Immunogenicity of BNT162b2 vaccine Against the Alpha and Delta Variants in Immunocompromised Patients. medRxiv 2021, posted 9 August. Full text: https://doi.org/10.1101/2021.08.08.21261766
Prospective study in 64 patients with systemic inflammatory diseases and 21 controls receiving two doses of the BioNTech/Pfizer vaccine. The Delta variant fully escaped the humoral response of individuals treated with rituximab. See also how they differentially impacted the immunogenicity of the BioNTech/Pfizer vaccine, by impairing B cell (rituximab) and T cell (methotrexate) responses.
11 August
Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years — COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. ePub: 6 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7032e3
Among adults aged ≥ 65, the effectiveness of full vaccination for preventing hospitalization was 96% for the BioNTech/Pfizer and Moderna vaccines (exception: 91% for the BioNTech/Pfizer vaccine in age ≥ 75 years) and 84% for the Janssen vaccine. Note that these data are from the pre-Delta era.
Nirenberg E. Myocarditis and COVID-19 mRNA vaccines. Deplatformdisease.com 2021, published 10 July. https://www.deplatformdisease.com/blog/myocarditis-and-covid-19-mrna-vaccines?format=amp
Not a scientific paper, but worth reading. The author mentions that compared to mRNA vaccine-induced myocarditis, leaving children unprotected or incompletely protected from COVID-19 is currently the bigger risk.
Pepe S, Gregory AT, Denniss AR. Myocarditis, Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart Lung Circ. 2021 Jul 30:S1443-9506(21)01156-2. PubMed: https://pubmed.gov/34340927. Full text: https://doi.org/10.1016/j.hlc.2021.07.011
Among 2,000,287 individuals receiving at least one dose of vaccine, 20 had vaccine-related myocarditis (1.0/100,000; median age: 36) and 37 had pericarditis (1.8/100,000; median age: 59). Myocarditis occurred a median of 3.5 days after vaccination, pericarditis developed after a median of 20 days. All patients were discharged after a median of 1 to 2 days. No one died.
10 August
Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatology 2021, published 6 August. Full text: https://doi.org/10.1016/S2665-9913(21)00222-8
Two prospective studies of 3682 patients with rheumatic diseases, 546 patients with multiple sclerosis, and 1147 healthy controls. Seroconversion after first vaccination was significantly lower in patients than in controls, but after the second vaccination, seroconversion exceeded 80% in all patient treatment subgroups, except among those treated with anti-CD20 therapies (three [43%] of seven patients). We all know the final message: don’t delay the second shot in patients receiving immunosuppressive drugs.
9 August
Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination — Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. ePub: 6 August 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7032e1
The immunological response elicited by vaccines might be better than the response elicited by natural SARS-CoV-2 infection. In Kentucky residents infected by COVID in 2020, those who were not vaccinated had a 2.34 times higher risk of reinfection than those who were fully vaccinated.
Perry RJ, Tamborska A, Bhagteshwar S, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet 2021, published 6 August. Full text: https://doi.org/10.1016/S0140-6736(21)01608-1
An analysis of 95 patients from more than 40 hospitals across the UK. Seventy patients had vaccine-induced immune thrombotic thrombocytopenia (VITT) and 25 did not. Patients with VITT-associated cerebral venous thrombosis had more intracranial veins thrombosed than non-VITT patients; they also more frequently had extracranial thrombosis. Death or dependency occurred in 47% of patients with VITT-associated cerebral venous thrombosis. Non-heparin anticoagulants and immunoglobulin treatment might improve VITT outcome.
Callaway E. COVID vaccine boosters: the most important questions. Nature 2021, published 5 August. Full text: https://www.nature.com/articles/d41586-021-02158-6
“Concerns over waning immunity and SARS-CoV-2 variants have convinced some countries to deploy extra vaccine doses — but it’s not clear to scientists whether most people need them.”
8 August
Public Health England 202100806. SARS-CoV-2 variants of concern and variants under investigation in England | Technical briefing 20. UK Government 2021, 6 August. https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201 | PDF: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1009243/Technical_Briefing_20.pdf
Again, for Delta variant infections, the viral load cycle threshold (Ct) is described as being similar for unvaccinated individuals (17.8) and those with a full vaccination schedule (18.0). The authors conclude that “similar Ct values (…) suggest limited difference in infectiousness.” Note that there is still the possibility that vaccinated people shed the virus for a shorter period of time. Vaccination may also reduce an individual’s overall risk of becoming infected. In any case, vaccination should be expected to reduce SARS-CoV-2 transmission, even of the Delta variant. Interesting discussions ahead.
Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021, published 6 August. Full text: https://doi.org/10.1016/S0140-6736(21)01694-9
BioNTech/Pfizer vs AstraZeneca – the first immunogenicity comparison of heterologous vaccine schedules. After measuring the SARS-CoV-2 anti-spike IgG concentrations (measured by ELISA) at 28 days after the vaccine boost, the authors find the following values for homologs and heterologs schedules (BNT: BioNTech/Pfizer; AZ: AstraZeneca):
BNT/BNT: 14,080 ELU/mL
AZ/BNT: 12,906 ELU/mL
BNT/AZ: 7133 ELU/mL
AZ/AZ: 1392 ELU/mL
Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021 Aug 3:S1074-7613(21)00303-4. PubMed: https://pubmed.gov/34348117. Full text: https://doi.org/10.1016/j.immuni.2021.07.017
SARS-CoV-2 vaccines have been developed and approved in time frames never before seen, a feat that bodes well for future advancements in medicine, not only in infectious diseases. The authors review the milestones, methods and outcomes of this effort and provide a perspective for how partnership and preparedness can be better utilized in response to future public-health pandemic emergencies.
7 August
Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv 2021, posted 5 August. Full text: https://doi.org/10.1101/2021.08.03.21261496
A retrospective study from Israel describing 33,993 fully vaccinated adults tried to answer one of the bigger questions these days: is the amount of time since the second injection of the BioNTech/Pfizer vaccine significantly associated with a risk of post-vaccination COVID-19 infection? Yes, it is. Those who received their second dose of vaccine at least 146 days before a new RT-PCR test (Group 1) had a higher risk of infection that those who received their vaccine less than 146 days before (Group 2). The absolute numbers are somewhat less scary: among people older than 60, 182/7021 (2.6%) tested positive in Group 1 and 19/2164 (0.9%) in Group 2.
Elliott P, Haw D, Wang H, et al. REACT-1 round 13 final report: exponential growth, high prevalence of SARS-CoV-2 and vaccine effectiveness associated with Delta variant in England during May to July 2021. Imperial College London 2021, published 4 August. https://spiral.imperial.ac.uk/handle/10044/1/90800
First, the good news: fully vaccinated people have lower viral loads (median Ct: 27.6) than unvaccinated or partially vaccinated people (23.1), so vaccines are likely to decrease the potential for the transmission of the Delta variant. Now, the bad news: between 24 June and 12 July 2021, with the Delta variant already firmly established in the UK, 44% of infections occurred in fully vaccinated individuals. The authors estimate vaccine effectiveness against infection to be 49%. Sex, ethnicity, household size and local levels of economy also jointly contributed to the risk of higher prevalence. The authors anticipate that increased mixing during the autumn in the presence of the Delta variant may lead to a new wave of the pandemic, even at high levels of vaccination. [Note that men had higher odds of infection than women, a finding not seen in a previous analysis (20 May and 7 June). The reason? Increased social mixing during England’s progression in the Euro 2020 football competition during June and July 2021…]
6 August
Zhang H, Liu Y, Liu D, et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 2021 Aug 2. PubMed: https://pubmed.gov/34341489. Full text: https://doi.org/10.1038/s41422-021-00541-6
Should you better go early in the morning to get your vaccine shot? The authors analyzed 63 health care workers who received the inactivated BBIBP-CorV vaccine (Sinopharm) either between 9 and 11 o’clock in the morning or between 3 and 5 o’clock in the afternoon. Strikingly, participants vaccinated in the morning showed significantly higher level of NAbs in the sera, 34.70 vs 19.35. Early birds also had stronger B cell and Tfh responses. Interesting path to follow.
Keeton R, Richardson SI, Moyo-Gwete T, et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant dependent manner. medRxiv 2021, posted 28 July. Full text: https://www.medrxiv.org/content/10.1101/2021.07.24.21261037v1
Therefore, vaccination with the J&J vaccine following previous infection, even > 6 months previously, may result in substantially enhanced protection against COVID-19. The authors conclude that this finding may be of particular relevance in settings of high SARS-CoV-2 seroprevalence.
Emanuel EJ, Skorton DJ. Mandating COVID-19 Vaccination for Health Care Workers. Ann Intern Med. 2021 Jul 30. PubMed: https://pubmed.gov/34328365. Full text: https://doi.org/10.7326/M21-3150
The Delta variant is paving the way for mandating COVID-19 vaccination, not only for health care workers.
5 August
Hause AM, Gee J, Baggs J, et al. COVID-19 Vaccine Safety in Adolescents Aged 12–17 Years — United States, December 14, 2020–July 16, 2021. MMWR Morb Mortal Wkly Rep. ePub: 30 July 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7031e1
As of July 16, 2021, almost 9 million US adolescents aged 12–17 years had received the BioNTech/Pfizer vaccine. The Vaccine Adverse Event Reporting System (VAERS) received 9246 reports, 90.7% of which were for non-serious adverse events while 9.3% were for serious adverse events, including around 400 cases of myocarditis (4.3%; about 1:25,000 vaccinees; see also https://www.cdc.gov/mmwr/volumes/70/wr/mm7027e2.htm). Systemic reactions were more common after the second dose.
Pinto D, Sauer MM, Czudnochowski N, et al. Broad betacoronavirus neutralization by a stem helix–specific human antibody. Science 2021, 3 August. Full text: https://science.sciencemag.org/content/early/2021/08/03/science.abj3321
On our way to a universal betacoronavirus vaccine? The authors describe 5 monoclonal antibodies (mAbs) cross-reacting with the stem helix of multiple β coronavirus spike glycoproteins isolated from COVID-19 convalescent individuals.
4 August
Chia PY, Ong S, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv 2021, posted 31 July. Full text: https://doi.org/10.1101/2021.07.28.21261295
Singapore, 218 patients admitted to hospital with Delta (B.1.617.2) SARS-CoV-2 infection. Vaccination (mostly with the BioNTech/Pfizer or Moderna vaccines) was associated with a faster decline in RNA viral load. The odds of severe COVID-19 requiring oxygen supplementation was significantly lower following vaccination (adjusted odds ratio 0.07, p = 0.001). PCR cycle threshold (Ct) values were similar between vaccinated and unvaccinated groups at diagnosis, but viral loads decreased faster in vaccinated individuals.
Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 11, 136 (2021). https://doi.org/10.1038/s41408-021-00528-x
In 267 patients with B cell chronic lymphocytic leukemia (CLL), one dose of vaccine generated detectable spike-specific antibody responses in 34% of patients with CLL compared to 94% of healthy donors. After the second dose of vaccine, antibody responses increased to 75% (n = 55), but titers remained lower than in controls. Current treatment with Bruton’s tyrosine kinase (BTK) inhibitors or IgA deficiency were independently associated with failure to generate an antibody response after the second vaccine.
Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med (2021). https://doi.org/10.1038/s41591-021-01469-5
In 910 adults with autoimmune rheumatic diseases (ARD) vaccinated with the Chinese CoronaVac vaccine, all had lower IgG seroconversion rates, and lower neutralizing antibody titers were lower than in 182 age- and sex-matched healthy adults.
3 August
DREES 20210729. Entrées hospitalières et décès de patients Covid-19 selon le statut vaccinal et la présence de la mutation L452R. DREES 2021 (Direction de la recherche, des études, de l’évaluation et des statistiques), published 29 July. Full text : https://solidarites-sante.gouv.fr/IMG/pdf/2021-07-23_-_sivic-sidep-vacsi_premiers_resultats_-_drees-2.pdf
In France, between 31 May and 11 July 2021, unvaccinated people represented around 85% of COVID-19 patients hospitalized, both in ICU units and non-ICU units. Fully vaccinated patients made up only 7% of admissions.
Merrill ED, Kashem SW, Amerson EH, et al. Association of Facial Pustular Neutrophilic Eruption With Messenger RNA-1273 SARS-CoV-2 Vaccine. JAMA Dermatol. 2021 Jul 28. PubMed: https://pubmed.gov/34319363. Full text: https://doi.org/10.1001/jamadermatol.2021.2474
The authors report a facial eruption that developed within 24 hours after receiving the Moderna vaccine in 2 patients without a history of known allergies, rosacea, facial/dental fillers, or prior SARS-CoV-2 infection.
2 August
Corbett KS, Nason MC, Flach B, et al. Immune Correlates of Protection by mRNA-1273 Immunization against SARS-CoV-2 Infection in Nonhuman Primates. Science 2021, published 29 July. PubMed: https://pubmed.gov/33907752. Full text: https://science.sciencemag.org/content/early/2021/07/29/science.abj0299
In Rhesus macaques, viral replication was significantly reduced in bronchoalveolar lavages and nasal swabs following a SARS-CoV-2 challenge in vaccinated animals and strongly correlated with levels of anti-S antibody and neutralizing activity. Lower antibody levels were needed for reduction of viral replication in the lower airway than in the upper airway.
Fallah M. Remember Ebola: stop mass death in Africa. Nature. 2021 Jul;595(7869):627. PubMed: https://pubmed.gov/34316054. Full text: https://doi.org/10.1038/d41586-021-01964-2
“Rich countries are hoarding vaccines, allowing doses to expire while unvaccinated people who want to be immunized die.”
1 August
Krantz MS, Kwah JH, Stone CA Jr, et al. Safety Evaluation of the Second Dose of Messenger RNA COVID-19 Vaccines in Patients With Immediate Reactions to the First Dose. JAMA Intern Med. 2021 Jul 26. PubMed: https://pubmed.gov/34309623. Full text: https://doi.org/10.1001/jamainternmed.2021.3779
All 159 patients with an immediate allergic reaction to the first dose of the Pfizer/BioNTech or Moderna vaccine, including 19 individuals with first-dose anaphylaxis, tolerated the second dose. (Antihistamine pre-medication had been given to 47 patients [30%] before the second dose). Thirty-two patients (20%) reported immediate and potentially allergic symptoms that were associated with the second dose that were self-limiting, mild, and/or resolved with antihistamines alone.
Stampfer SD, Goldwater MS, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia (2021). Full text: https://doi.org/10.1038/s41375-021-01354-7
Study of 103 multiple myeloma (MM) patients (96 patients with active MM and 7 with smoldering (asymptomatic) disease). Smoldering MM patients responded better than those with active disease. Only 45% of active MM patients developed an adequate response, while 22% had a partial response. Lower spike antibody levels were associated with older age, impaired renal function, low lymphocyte counts, reduced uninvolved immunoglobulin levels, > second line of treatment, and among those not in complete remission.
31 July
Wu S, Huang J, Zhang Z, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Resp Med 2021, published 26 July. Full text: https://doi.org/10.1016/S1473-3099(21)00396-0
Two doses of an aerosolized adenovirus type-5 vector-based COVID-19 vaccine – equivalent to a fifth or two-fifths of an intramuscular dose – were well-tolerated and did not produce serious side effects in healthy adults. They elicited strong IgG and neutralizing antibody responses similar to one dose of an intramuscular injection.
Bhuyan P, Medin J, Gomes da Silva H. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: a global safety database analysis. Lancet 2021, published 27 July. Full text: https://doi.org/10.1016/S0140-6736(21)01693-7
Vaccine-induced immune thrombotic thrombocytopenia (VITT or TTS) seems to be rare after the second dose of the AstraZeneca vaccine. A short description of 13 cases: 8 individuals with pulmonary embolism, co-occurring with cerebral venous sinus thrombosis (CVST) in two individuals; one individual with CVST occurring alone; one individual with deep vein thrombosis; one individual with thrombotic stroke; and two individuals with unspecified embolisms.
30 July
Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021 Jul 28. PubMed: https://pubmed.gov/34320281. Full-text: https://doi.org/10.1056/NEJMoa2109072
Long COVID after breakthrough infections? Most breakthrough infections during the 4-month period after the second vaccine dose (39/1497, 0.4%) were mild or asymptomatic; however, 7 patients (19%) had persistent symptoms (> 6 weeks), including headaches, muscle pain, loss of taste and smell and fatigue), indicating that Long COVID may occur among people who experience breakthrough infections. Note that the study is from the pre-Delta world (20 January through 28 April; 85% of the samples yielded the Alpha strain [B.1.1.7]). Expect the data to be less favorable in settings where Delta (B.1.617.2) is the dominant strain. It may be wise to follow the new CDC guidelines and wear masks indoors in risky situations (see the next paragraph, “Prevention”).
Thomas SJ, Moreira ED, Kitchin N, et al. Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. medRxiv 2021, posted 28 July. Full text: https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1
Six-month efficacy data from the Pfizer/BioNTech vaccine trial: 1) Efficacy declining from 96% at 2 months to 85% at 4-6 months for infection, for an average decline of ~6% every two months; 2) Protection against severe illness was 97% through at least 6 months.
29 July
Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Regional Health Europe 2021, published 22 July. Full text: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(21)00155-1/fulltext
A prospective study exploring the eight-week time course of specific cellular or/and humoral immune responses after two doses of vaccine. Seroconversion efficacy in dialysis patients was similar to medical personnel (> 95%), but markedly impaired in 368 kidney transplant recipients (42%).
Ikegame S, Siddiquey M, Hung CT, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Res Sq. 2021. PubMed: https://pubmed.gov/33851150. Full-text: https://www.nature.com/articles/s41467-021-24909-9
In Argentina, sera from Sputnik vaccine recipients had a median 6.1-fold and 2.8-fold reduction in GMT against B.1.351 and the E484K mutant spike, respectively. “When extrapolated to full serum strength, half of the sera samples failed to achieve an IC80 and only 1 out 12 achieved an IC90 against B.1.351.” The authors conclude that “control of some emergent SARS-CoV-2 variants may benefit from updated vaccines.”
28 July
Paetzold J, Krammer F, van Laer D, et al. The effects of rapid mass vaccination against SARS-CoV-2 and its Variants-of-Concern: Evidence from an early VoCs hotspot. Research Square 2021, posted 24 July. Full text: https://doi.org/10.21203/rs.3.rs-741944/v1
Following a large outbreak of the Beta (B.1.351, “South Africa”) and Alpha variants (B.1.1.7/E484K, “England”) in the district of Schwaz, Austria, more than 70% of the adult population of the district received their first Pfizer/BioNTech vaccine dose within 6 days (11 and 16 March). Result: a 60% reduction in new SARS-CoV-2 infections relative to people residing just outside of the vaccinated district.
Lund FE, Randall TD. Scent of a vaccine. Science 2021, published 23 July. Full text: https://science.sciencemag.org/content/373/6553/397
About the advantages of intranasal vaccines: needle-free administration, on-site delivery and mucosal immunity.
27 July
Arunachalam PS, Scott MKD, Hagan T, et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021 Jul 12. PubMed: https://pubmed.gov/34252919. Full-text: https://doi.org/10.1038/s41586-021-03791-x
Booster injections of mRNA vaccines might stimulate a “strikingly enhanced innate immune response” compared to the primary injection.
26 July
Massa F, Cremoni M. Safety and Cross-Variant Immunogenicity of a Three-Dose COVID-19 mRNA Vaccine Regimen in Kidney Transplant Recipients. Lancet Preprints 2021, posted 22 July. Full text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3890865
Important study from Nice, France. In kidney transplant recipients (n = 61), a three-dose BioNTech/Pfizer vaccine regimen led to an increase in spike-specific IgG levels, neutralizing activity and number of IFN-γ-secreting cells. However, neutralizing antibody titers remained low even after three doses, especially against new variants. The authors recommend continuation of barrier measures and vaccination of relatives.
Benotmane I, Gautier G, Perrin P, et al. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA. 2021 Jul 23. PubMed: https://pubmed.gov/34297036. Full-text: https://doi.org/10.1001/jama.2021.12339
Same message as above, now from Strasbourg, France. In kidney transplant recipients (n = 159), a third dose of the Moderna vaccine induced a serologic response in 49% of patients who did not respond after 2 doses. Patients taking tacrolimus, mycophenolate, and steroids were less likely to develop anti–SARS-CoV-2 antibodies than those treated with other regimens (35% vs 63%).
Robinson KA, Maimone S, Gococo-Benore DA, Li Z, Advani PP, Chumsri S. Incidence of Axillary Adenopathy in Breast Imaging After COVID-19 Vaccination. JAMA Oncol. 2021 Jul 22. PubMed: https://pubmed.gov/34292295. Full-text: https://doi.org/10.1001/jamaoncol.2021.3127
In this retrospective analysis (n = 750), 23 (3%) patients had axillary adenopathy on mammography. This incidence is higher than axillary adenopathy in otherwise normal mammography (0.02% – 0.04%). The incidence of adenopathy decreased over time with no adenopathy seen in patients who received the vaccine more than 28 days previously. When possible, consider scheduling screening breast imaging 4 to 6 weeks after the second COVID-19 vaccination dose (National Comprehensive Cancer Network, 15 June 2021, Recommendations of the NCCN COVID-19 Vaccination Advisory: https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v3-0.pdf
25 July
Mateo-Urdiales A, Spila Alegiani S, Fabiani M, et al. Risk of SARS-CoV-2 infection and subsequent hospital admission and death at different time intervals since first dose of COVID-19 vaccine administration, Italy, 27 December 2020 to mid-April 2021. Euro Surveill. 2021 Jun;26(25):2100507. PubMed: https://pubmed.gov/34169819. Full-text: https://doi.org/10.2807/1560-7917.ES.2021.26.25.2100507
A report on 7,370,008 persons who had received their first dose of vaccine by 4 April 2021 in Italy. As in earlier studies from Israel, a partial protection can be seen as early as two weeks after the first dose.
Mazagatos C, Monge S, Olmedo C, et al. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021 Jun;26(24):2100452. PubMed: https://pubmed.gov/34142647. Full-text: https://doi.org/10.2807/1560-7917.ES.2021.26.24.2100452
The same message from a group of elderly residents (aged 65 years and older) in long-term care facilities: partial vaccine effectiveness (VE) of 50% with the first dose of the BioNTech/Pfizer and the Moderna vaccine. After the second dose, VE was 71% against SARS-CoV-2 infection, 88% and 97% against COVID-19 hospitalizations and deaths, respectively.
DiPiazza AT, Leist SR, Abiona OM, et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. 2021 Jul 2:S1074-7613(21)00262-4. PubMed: https://pubmed.gov/34270939. Full-text: https://doi.org/10.1016/j.immuni.2021.06.018
The title is the message: vaccination with the Moderna vaccine (mRNA-1273) does not result in disease enhancement following infection with SARS-CoV-2.
24 July
Wei J, Stoesser N, Matthews PC, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021 Jul 21. PubMed: https://pubmed.gov/34290390. Full-text: https://doi.org/10.1038/s41564-021-00947-3
In this UK cohort of 45,965 adults, seroconversion rates and antibody levels after a single vaccine dose were lower in older individuals, especially in those aged > 60 years. Low responders were more common among people taking immunosuppressants or corticosteroids, as well as in patients with rheumatoid arthritis, chronic liver disease, cancer, type 2 diabetes, obesity, and asthma.
23 July
Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021 Jul 21. PubMed: https://pubmed.gov/34289274. Full-text: https://doi.org/10.1056/NEJMoa2108891
Effectiveness after one dose of the BioNTech/Pfizer or AstraZeneca was lower among individuals infected with the Delta variant (B.1.671.2, “India”) than with the Alpha variant (B.1.1.7, “England”): Delta, 30.7% – Alpha, 48.7% (there was no difference between the two vaccines). After two doses, differences in vaccine effectiveness with the two variants were modest, but the BioNTech/Pfizer vaccine was superior to the AstraZeneca vaccine (BioNTech/Pfizer: Delta, 88.0% – Alpha, 93.7%; AstraZeneca: Delta, 67.0%; Alpha, 74.5%).
22 July
Ledford H. Should children get COVID vaccines? What the science says. Nature 2021, published 20 July. Full text: https://www.nature.com/articles/d41586-021-01898-9
Vaccinating children. Is it necessary? Is it safe? And how will it affect the pandemic?
Mateus J, Dan JM, Zhang Z, et al. Low dose mRNA-1273 COVID-19 vaccine generates durable T cell memory and antibodies enhanced by pre-existing crossreactive T cell memory. medRxiv 2021, posted 5 July. Full text: https://doi.org/10.1101/2021.06.30.21259787
At 25 µg (instead of the four-fold higher 100 µg standard dose), the Moderna vaccine mRNA-1273 vaccine induced a durable and functional T cell and antibody response comparable to natural infection. If these preliminary findings are confirmed, the lower dose could accelerate the global immunization campaign (and perhaps help with side effects?).
21 July
Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity in uninfected and previously infected individuals. medRxiv 2021, posted 18 July. Full text: https://www.medrxiv.org/content/10.1101/2021.07.14.21260307v1
Analysis of plasma neutralization using 16 SARS-CoV-2 variants. The Beta (B.1.351, ‘South Africa’) and Gamma (P.1, ‘Brazil’) strains, showed the greatest reduction, followed by the Delta (B.1.617.2, ‘India’) and Alfa (B.1.1.7, ‘England’) strains. Plasma from previously infected vaccinated individuals had a better neutralization than plasma from uninfected vaccinated individuals.
Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully-vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021 Jul 7:S1198-743X(21)00367-0. PubMed: https://pubmed.gov/34245907. Full-text: https://doi.org/10.1016/j.cmi.2021.06.036
Retrospective study of 152 individuals who developed COVID-19 more than 7 days after the second BioNTech/Pfizer vaccine dose and required hospitalization. High rate of existing co-morbidities: hypertension (71%), diabetes (48%), congestive heart failure (27%), lung diseases (24%), chronic kidney (24%), dementia (19%) and cancer (24%). Importantly, 60 (40%) patients were immunocompromised. Thirty-four (22%) patients died.
Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting. Ann Intern Med 2021, published 20 July. Full text: https://doi.org/10.7326/M21-1577
In this large US veteran cohort (> 108,000), the overall vaccine effectiveness was 97% seven or more days after the second dose of the mRNA vaccine.
20 July
Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021, published 15 July. Full text: https://doi.org/10.1016/S2666-5247(21)00177-4
Preliminary data from a study by Malik Peiris et al., one of the central figures of the 2002/2003 SARS-CoV-1 epidemic. Health-care workers in Hong Kong who received the BioNTech-Pfizer vaccine (n = 63) had higher antibody concentrations and higher PRNT50 (plaque reduction neutralization test) titers than those who received the Sinovac vaccine (n = 30). The authors conclude that “the difference in concentrations of neutralizing antibodies identified in our study could translate into substantial differences in vaccine effectiveness”.
18 July
Barouch DH, Stephenson KE, Sadoff J, et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. NEJM July 14, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2108829?query=featured_home
This small study indicates that the Ad26.COV2.S vaccine (J&J) elicited durable humoral and cellular immune responses with minimal decreases for at least 8 months. Moreover, there was an expansion of neutralizing antibody breadth associated with improved coverage of SARS-CoV-2 variants over time, including increased neutralizing antibody titers against Delta.
17 July
Normark J, Vikström L, Gwon YD, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. NEJM July 14, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2110716?query=featured_home
This relatively small cohort suggests that the mRNA-1273 vaccine (MODERNA) boost may provide better protection against the B.1.351 variant than a ChAdOx1 nCoV-19 boost. However, the mRNA-1273 boost led to more frequent reports of fever, headache, chills, and muscle aches than the ChAdOx1 nCoV-19 boost.
Powell AA, Power L, Westrop S, et al. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Euro Surveill. 2021;26(28):pii=2100634. https://doi.org/10.2807/1560-7917.ES.2021.26.28.2100634
Same direction. After the second vaccine dose, previously uninfected adults in both heterologous vaccination groups had significantly higher reactogenicity than their homologous counterparts, with similar rates among those receiving ChAd/BNT (54.4%) and BNT/ChAd (55.2%) compared with ChAd/ChAd (33.5%) or BNT/BNT (33.3%). Severe reactogenicity was 2.4 times (27.8% vs. 11.6%) more likely with heterologous regimens, including increased requirement for medical attention. These findings were irrespective of the reason for receiving a heterologous schedule.
16 July
Rossi AH, Ojeda DS, Varese A, et al. Sputnik V Vaccine Elicits Seroconversion and Neutralizing Capacity to SARS CoV-2 after a Single Dose. Cell Rep Med July 13, 2021. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(21)00208-1
Of 227 seronegative healthcare workers in Argentina, 94% developed spike-specific IgG antibodies at day 21 after the first dose. A single Sputnik V dose elicited higher antibody levels and virus neutralizing capacity in 67 previously infected individuals than in naïve ones receiving the full two-dose schedule.
15 July
Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med Jul 14, 2021. https://www.nature.com/articles/s41591-021-01449-9
ChAdOx1 (AstraZeneca/Oxford)-primed immune responses before and 3 weeks after booster with ChAd (n = 32) or BioNTech/Pfizer’s BNT162b2 (n = 55): although both vaccines boosted prime-induced immunity, BNT162b2 induced significantly higher frequencies of spike-specific CD4+ and CD8+ T cells and, in particular, high titers of neutralizing antibodies against the B.1.1.7, B.1.351 and P.1 variants.
13 July
Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatology July 08, 2021. https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(21)00212-5/fulltext
Seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression. In 84 patients with psoriasis receiving immunosuppressive drugs, seroconversion rates were lower in patients receiving immunosuppressants (78%), compared to 17 controls (100%), with the lowest rate in those receiving methotrexate (47%). However, cellular responses were preserved in almost all patients.
Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol July 8, 2021. https://jamanetwork.com/journals/jamaoncology/fullarticle/2781608?resultClick=1
This cohort study compared serologic status and safety of the BNT162b2 vaccine in 232 patients receiving active treatment for cancer and 261 health care workers who served as controls. After the first dose of the vaccine, only 29% of the patients were seropositive compared with 84% of the controls; after the second dose, the seropositive rate of the patients reached 86%, and reported adverse events resembled those of healthy individuals.
Vaccine
7 July
Tran KC, Andronico A, Bosetti P, et al. Benefits and risks associated with different uses of the COVID-19 vaccine Vaxzevria: a modelling study, France, May to September 2021. Euro Surveill. 2021;26(26):pii=2100533. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.26.2100533
A modeling study, indicating that for individuals 55 years and older, the benefits of distributing AstraZeneca’s Vaxzevria largely outweighs the risks in a range of possible scenarios. In contrast, in young adults, the risks were similar or higher than the benefits.
Sangli S, Virani A, Cheronis N, et al. Thrombosis With Thrombocytopenia After the Messenger RNA–1273 Vaccine. Annals Int Med, June 29, 2021. https://www.acpjournals.org/doi/10.7326/L21-0244
A 65-year-old man with vaccine-induced thrombosis with thrombocytopenia (VITT) developing a few days after administration of the mRNA-1273 vaccine from Moderna: most of his clotting and other relevant work-up was consistent with the syndrome. This report complicates the hypothesis that implicates adenoviral vectors as the sole cause of VITT or TTS.
6 July
Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Hematology July 02, 2021. https://www.thelancet.com/journals/lanhae/article/PIIS2352-3026(21)00169-1/fulltext
According to this large cohort, patients actively treated with BTKIs, ruxolitinib, venetoclax, or anti-CD20 antibody therapies seem to be the most negatively affected and unprotected from SARS-CoV-2 infection.
Nathan A, Rossin EJ, Kaseke C, et al. Structure-guided T cell vaccine design for SARS-CoV-2 variants and sarbecoviruses. Cell June 29, 2021. https://www.cell.com/cell/fulltext/S0092-8674(21)00797-2
The development of immunogens that can induce CD8+ T cell responses to mutationally constrained epitopes could greatly augment current vaccines for SARS-CoV-2 given the emergence of variants that escape convalescent plasma and vaccine-induced antibody responses. This study elucidates key mutationally constrained regions and immunogenic epitopes in the SARS-CoV-2 proteome for a global T cell-based vaccine against emerging variants and sarbecoviruses.
5 July
Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Resp Med Jul 2, 2021. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00220-4/fulltext
A lot of data from Israel. A high correlation (r=0.92) was seen between IgG against the receptor-binding domain and neutralizing antibody titers. First-dose induced IgG response was significantly lower in individuals aged 66 years and older and immunosuppressed individuals. Of note, this disparity was partly abrogated following the second dose.
4 July
Thompson MG, Burgess JL, Naleway AL, et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. NEJM June 30, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2107058?query=featured_home
This prospective cohort study involving 3975 health care personnel, first responders, and other essential and frontline workers shows that mRNA vaccines attenuated the viral RNA load, risk of febrile symptoms, and duration of illness among those who had breakthrough infection despite vaccination.
Ropper AH, Klein JP. Cerebral Venous Thrombosis. NEJM Jul 1, 2021. https://www.nejm.org/doi/full/10.1056/NEJMra2106545?query=featured_home
Nice review. Allan H. Ropper and Joshua P. Klein summarize current knowledge on how to diagnose and how to treat cerebral venous thrombosis.
Sánchez van Kammen M, Brodard J, Scutelnic A, et al. Frequency of Thrombocytopenia and Platelet Factor 4/Heparin Antibodies in Patients With Cerebral Venous Sinus Thrombosis Prior to the COVID-19 Pandemic. JAMA July 2, 2021. https://jamanetwork.com/journals/jama/fullarticle/2781791?resultClick=1
In 865 patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic, baseline thrombocytopenia was uncommon (8.4%), and heparin-induced thrombocytopenia (0.1%) and platelet factor 4/heparin antibodies were rare.
3 July
Heath PT, Galiza EP, Baxter DN, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. NEJM Jun 30, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2107659?query=featured_home
Paper of the day. The next vaccine! Large RCT of the NVX-CoV2373 vaccine (Novavax), a recombinant nanoparticle vaccine that contains the full-length spike glycoprotein of the prototype strain plus Matrix-M adjuvant. Among 14039 participants, the two-dose regimen conferred 89.7% protection. A post hoc analysis showed an efficacy of 86.3% against the B.1.1.7 (or alpha) variant and 96.4% against non-B.1.1.7 variants.
30 June
Jalkanen P, Kolehmainen P, Häkkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun June 28, 2021, 12, 3991. https://www.nature.com/articles/s41467-021-24285-4
After the second dose, the sera of BNT162b2-vaccinated health care workers (n = 180) from Finland effectively neutralized the SARS-CoV-2 variant with the D614G substitution and the B.1.1.7 variant, whereas the neutralization of the B.1.351 variant was five-fold reduced. However, 92% of the seronegative vaccinees had a neutralization titre of >20 for the B.1.351 variant indicating some protection.
27 June
Cai Y, Zhangf J, Xiao T, et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science 24 Jun 2021: eabi9745. https://science.sciencemag.org/content/early/2021/06/23/science.abi9745
How has SARS-CoV-2 evolved to enhance viral fitness and immune evasion? This study describes cryo-EM structures of the full-length spike (S) trimers of the B.1.1.7 and B.1.351 variants, as well as their biochemical and antigenic properties. Of note, findings also suggest that the most problematic combination of mutations is not (yet) present in the existing variants examined here.
Otto SP, Day T, Arino J, et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Current Biology June 23, 2021. https://doi.org/10.1016/j.cub.2021.06.049
Nice overview about the evolutionary processes involved in the emergence and selection of new variants of concern (VOC), about what we can expect in terms of the future emergence of VOCs, and what we can do to minimize their impact.
Jennewein MF, MacCamy AJ, Atkins NR, et al. Isolation and Characterization of Cross-Neutralizing Coronavirus Antibodies from COVID-19+ Subjects. Cell Rep June 21, 2021. https://doi.org/10.1016/j.celrep.2021.109353
Among 198 antibodies isolated from four COVID-19+ subjects, four cross-neutralizing antibodies neutralized the B.1.351 mutant strain.
Bugembe DL, Phan MV, Ssewanyana I, et al. Emergence and spread of a SARS-CoV-2 lineage A variant (A.23.1) with altered spike protein in Uganda. Nat Microbiol June 23, 2021. https://www.nature.com/articles/s41564-021-00933-9
A new variant of concern? The authors describe the emergence and spread of a SARS-CoV-2 variant of the A lineage (A.23.1) with multiple protein changes throughout the viral genome. A.23.1 shares many features found in the lineage B VOCs, including alteration of key spike protein regions, especially the ACE 2 binding region, the furin cleavage site and the 613/614 change.
McEwen AE, Cohen S, Bryson-Cahn C, et al. Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State. Clinical Infectious Diseases June 24, 2021, ciab581, https://doi.org/10.1093/cid/ciab581
Across 20 vaccine breakthrough cases detected, all 20 were due to variants of concern (VOC) and had a low median Ct of 20.2 (IQR=17.1-23.3). Variants B.1.427, B.1.429, and B.1.1.7 were 3.38-fold, 1.51-fold, and 1.29-fold more common in breakthrough cases compared to controls.
25 June
Shroti M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Inf Dis June 23, 2021. https://doi.org/10.1016/S1473-3099(21)00289-9
Among 10,412 care home residents aged 65 years and older, adjusted hazard ratios (HRs) for PCR-positive infection relative to unvaccinated residents declined from 28 days after the first vaccine dose to 0.44 (95% CI 0.24–0.81) at 28–34 days and 0.38 (0.19–0.77) at 35–48 days. Similar effect sizes were seen for ChAdOx1 and BNT162b2 vaccines at 35–48 days.
Hyams C, Marlow R, Maseko Z. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Inf Dis June 23, 2021. https://doi.org/10.1016/S1473-3099(21)00330-3
Same direction. One dose of either BNT162b2 or ChAdOx1 nCoV-19 resulted in substantial risk reductions of COVID-19-related hospitalisation in people aged at least 80 years. The adjusted vaccine effectiveness was 80.4% (95% CI 36.4–94.5).
Prendecki M, Willicombe M. Single-dose SARS-CoV-2 vaccination efficacy in the elderly. Lancet Inf Dis 2021, June 23. https://doi.org/10.1016/S1473-3099(21)00354-6
In their comment on the two studies above, Maria Prendecki and Michelle Willicombe argue that both studies give cause for optimism; despite older individuals developing decreased humoural responses to vaccines, including SARS-CoV-2, vaccine efficacy is high, and second doses will probably increase efficacy further. The results also highlight some of the difficulties in trying to make comparisons between vaccines in real-world studies.
24 June
Bøhler AD, Strøm ME, Sandvig KU et al. Acute macular neuroretinopathy following COVID-19 vaccination. Eye June 21, 2021. https://www.nature.com/articles/s41433-021-01610-1
A patient who developed an acute paracentral scotoma after having received the AstraZeneca vaccine: the signs and symptoms were consistent with acute macular neuroretinopathy (AMN).
Matheney M, Maleque N, Channell N, et al. Severe Exacerbations of Systemic Capillary Leak Syndrome After COVID-19 Vaccination: A Case Series. Ann Int Med June 15, 2021. https://doi.org/10.7326/L21-0250
Three patients who had severe flares of SCLS immediately after receiving standard doses of the COVID-19 vaccines (AstraZeneca, BioNTech and Moderna). The authors recommend that patients with a diagnosis or suspected diagnosis of SCLS should receive IVIG prophylaxis before vaccination.
23 June
Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Int Med 15 June, 2021. https://www.acpjournals.org/doi/10.7326/L21-0282
A booster may work. In 30 patients, antibody titers increased after the third dose in one third of patients who had negative antibody titers and in all patients who had low-positive antibody titers. Vaccine reactions seem to be acceptable.
22 June
Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA Vaccines in Patients with Cancer. Cancer Cell June 18, 2021. https://doi.org/10.1016/j.ccell.2021.06.009
None of the patients with a history of anti-CD20 antibody in the 6 months prior to vaccination developed an antibody response. None of the four patients with a history of anti-CD20 antibody in the 6 months prior to vaccination developed an antibody response.
Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV, June 18, 2021. https://doi.org/10.1016/S2352-3018(21)00103-X
In this study of 53 people with HIV, ChAdOx1 nCoV-19 was safe and immunogenic. There was no correlation between the magnitude of the anti-spike IgG response at day 56 and CD4 cell count or age.
20 June
Stankov MV, Cossmann A, Bonifacius A, et al. Humoral and cellular immune responses against SARS-CoV-2 variants and human coronaviruses after single BNT162b2 vaccination. Clin Inf Dis June 16, 2021, ciab555, https://doi.org/10.1093/cid/ciab555
This study shows suboptimal neutralizing antibody activity after a single BNT162b2 vaccination. T cells, which responded equally to spike-derived peptides from SARS-CoV-2 WT, B.1.1.7 and B.1.351 were detectable with a broad inter-individual range. The authors propose that non-neutralizing antibody function and/or cellular immunity constitute an important outcome after vaccination and may be part of the early defense against SARS-CoV-2 infection.
19 June
Gonzalez DC, Nassau DE, Khodamoradi K et al. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA June 17, 2021. https://jamanetwork.com/journals/jama/fullarticle/2781360?resultClick=1
No significant decreases (repeat: no significant decreases).
18 June
Today another vaccine special on effectiveness against the delta variant and in HCW, on delayed local reactions, as well as on safety and immunogenicity in pregnant women.
Safety
Samarakoon U, Alvarez-Arango S, Blumenthal KG. Delayed Large Local Reactions to mRNA Covid-19 Vaccines in Blacks, Indigenous Persons, and People of Color. NEJM June 9, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2108620?query=featured_home
Photos of eight large local reactions are shown. The mean time from vaccination until the onset of the reaction was 8 ± 2 days (range, 4 to 14).
Effectiveness
Gupta K, O’Brian WJ, Bellino P, et al. Incidence of SARS-CoV-2 Infection in Health Care Workers After a Single Dose of mRNA-1273 Vaccine. JAMA Netw Open June 16, 2021;4(6):e2116416. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2781173?resultClick=1
This study demonstrated an association between receipt of the mRNA-1273 vaccine and a reduction in SARS-CoV-2 infection in HCWs beginning 8 days after dose 1. Vaccine clinical effectiveness was 50.3% for the entire 42-day period of follow-up, 77.5% for days 8 through 42, and 95.0% for days 15 through 42.
Stowe J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1617.2) variants. June 14, 2021. https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view/479607266
Not peer-reviewed, but incredibly good news and very important for everybody who is talking every day with hundreds of patients about the effectiveness of the AstraZeneca vaccine. Yes, they can take their second shot! There is no need to switch. Among 14,019 symptomatic cases infected with the delta variant (first seen in India), the Pfizer/BioNTech vaccine was 96% effective against hospitalization after 2 doses. Of note, the AstraZeneca vaccine was 92% effective (71% after 1 dose) against hospitalization after 2 doses. These rates were comparable to alpha variants.
Immunogenecity
Demonbreun AR, Sancilio A, Velez ME, et al. COVID-19 mRNA vaccination generates greater IgG levels in women compared to men. J Inf Dis June 12, 2021, jiab314, https://doi.org/10.1093/infdis/jiab314
In community participants with no prior history of COVID-19, women had higher levels of mean anti-RBD IgG after vaccination compared to men. After two doses, IgG levels remained significantly higher for women (30.4 µg/ml) compared to men (20.6 µg/ml), while percent inhibition was similar (98.4% vs 97.7%).
Pregnancy
Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA June 1´5 2021; 325(23):2370-2380. https://jamanetwork.com/journals/jama/fullarticle/2780202?resultClick=1
In this study of 103 pregnant and lactating women, mRNA vaccines were immunogenic in and induced immune responses against SARS-CoV-2 variants.
Razzaghi H, Meghani M, Pingali C, et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy — Eight Integrated Health Care Organizations, United States, December 14, 2020–May 8, 2021. MMWR Morb Mortal Wkly Rep. ePub: 15 June 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7024e2.htm?s_cid=mm7024e2_w
As of May 8, 2021, 16.3% of pregnant women identified in the CDC Datalink had received ≥ 1 dose of a COVID-19 vaccine during pregnancy in the US. Vaccination was lowest among Hispanic (11.9%) and non-Hispanic Black women (6.0%) and women aged 18–24 years (5.5%) and highest among non-Hispanic Asian women (24.7%) and women aged 35–49 years (22.7%).
17 June
Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ June 14, 2021; 373 doi: https://doi.org/10.1136/bmj.n1435
Large variations in the observed rates of 15 pre-specified AESIs (stroke, thrombosis, myocarditis, narcolepsy, myelitis, etc) by age and sex, showing the need for stratification or standardization before using background rates for safety surveillance. Considerable population level heterogeneity in AESI rates was found between databases.
16 June
Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med June 14, 2021. https://www.nature.com/articles/s41591-021-01413-7
Analyzing 813 viral genome sequences, the authors showed that vaccinees who tested positive at least 7 days after the second dose were disproportionally infected with B.1.351, compared with controls. Those who tested positive between 2 weeks after the first dose and 6 days after the second dose were disproportionally infected by B.1.1.7.
Pollett SD, Richard SA, Fries AC, et al. The SARS-CoV-2 mRNA vaccine breakthrough infection phenotype includes significant symptoms, live virus shedding, and viral genetic diversity. Clin Inf Dis June 12, 2021, ciab543. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab543/6297424
A total of 24 “VBI” (vaccine breakthrough infections) in predominantly young healthy persons. While none required hospitalization, a proportion experienced severe symptoms and shed live virus as high as 4.130 PFU/mL. Though of relatively low magnitude, the presence of infectious virus which may indicate a transmission risk of VBI. Infecting genotypes included both variants and wild type.
10 June
Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination amongst patients with cancer. Cancer Cell June 05, 2021. https://www.sciencedirect.com/science/article/pii/S1535610821002853
A high seroconversion rate (94%) in 200 vaccinated patients with cancer. Seroconversion was lower in recipients following highly immunosuppressive therapies such as anti-CD20 therapies (70%) and stem cell transplantation (73%) but not immune checkpoint inhibitor therapy (97%). IgG titers were lower following vaccination with the adenoviral than with the mRNA-based vaccines.
9 June
Chodick G, Tene K, Patalon T, et al. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw Open June 7, 2021; 4(6):e2115985. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2780700?resultClick=1
In this study of 503,875 individuals from Israel who received 1 dose of the BNT162b2 vaccine, the first dose was associated with an approximately 54% reduction in the risk of symptomatic SARS-CoV-2 infections at 13 to 24 days after immunization compared to 1 to 12 days after vaccination.
4 June
Takuva S, Takalani A, Garrett N, et al. Thromboembolic Events in the South African Ad26.COV2.S Vaccine Study. NEJM 3, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2107920?query=featured_home
Interim safety data from the first 288,368 participants who were vaccinated with Ad26.COV2.S (Johnson & Johnson) in the Sisonke study — an open-label, Phase IIIb implementation study. The rate of adverse events with vaccination is low, and thromboembolic events occur mainly in persons with risk factors for thromboembolism.
3 June
Martínez-Baz I, Miqueleiz A, Casado I, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill May 27, 2021 2021;26(21):pii=2100438. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.21.2100438
Does it make sense to vaccinate close contacts? Probably. Results of this study suggest a moderate effectiveness (35%) of BionTech/Pfizer and Astra Zeneca vaccines in preventing SARS-CoV-2 infection. Estimates increased against symptomatic and hospitalised COVID-19. Vaccine effectiveness was moderate in people with one dose (35%) and was higher after the second dose (66%), especially against hospitalisations (72% and 95%, respectively).
Vogel G, Couzin-Frankel J. Israel reports link between rare cases of heart inflammation and COVID-19 vaccination in young men. Nature NEWS June 1, 2021. https://www.sciencemag.org/news/2021/06/israel-reports-link-between-rare-cases-heart-inflammation-and-covid-19-vaccination
Brief summary of current knowledge: in Israel, between one in 3000 and one in 6000 men ages 16 to 24 who received the BioNTech vaccine developed myocarditis. Most cases were mild and resolved within a few weeks. It is speculated that very high antibody levels generated in young people may also, in rare cases, lead to a sort of immune overreaction that inflames the heart.
30 May
Frenck RW, Klein NP, Kitchin N, et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. NEJM May 27, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2107456?query=featured_home
In 1131 adolescents (12 to 15 years old), the BNT162b2 vaccine had a favorable safety profile. The immune response was non-inferior to that observed in the cohort of 16-to-25-year-old young adults.
28 May
Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. A Randomized Clinical Trial. JAMA May 26, 2021. https://jamanetwork.com/journals/jama/fullarticle/2780562?resultClick=24
Interim analysis of an RCT with 40,382 participants from countries in the Middle East who received at least 1 dose of a 2-dose inactivated vaccine series developed from either SARS-CoV-2 WIV04 or HB02 strains or an aluminum hydroxide–only control. Primary endpoint was the incidence of symptomatic COVID-19 at least 14 days after the second injection. The efficacy for the 2 vaccines was 72.8% in the WIV04 group and 78.1% in the HB02 group. Given the young age of the participants (median 36 years), there were only 2 severe infections, both occuring in the control arm.
Salzman MB, Huang C-W, O’Brien CM, Castillo RD. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis May 26, 2021. https://wwwnc.cdc.gov/eid/article/27/7/21-0594_article
Three patients from California are described, who experienced multisystem inflammatory syndrome (MIS) after immunization and SARS-CoV-2 infection.
27 May
CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC — United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. ePub: 25 May 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.htm?s_cid=mm7021e3_w
As of April 30, 2021, approximately 101 million persons in the US had been fully vaccinated against COVID-19. A total of 10,262 SARS-CoV-2 vaccine breakthrough infections had been reported, among them 6446 (63%) in females. In total, 995 (10%) patients were known to be hospitalized, and 160 (2%) patients died. Sequence data were available from 555 (5%) reported cases, 356 (64%) of which were identified as SARS-CoV-2 variants of concern, including B.1.1.7 (199; 56%), B.1.429 (88; 25%), B.1.427 (28; 8%), P.1 (28; 8%), and B.1.351 (13; 4%).
24 May
Formeister EJ, Chien W, Agrawal Y, et al. Preliminary Analysis of Association Between COVID-19 Vaccination and Sudden Hearing Loss Using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngology–Head & Neck Surgery, May 20, 2021. https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2780288?resultClick=1
No association exists between mRNA vaccines and sudden hearing loss.
23 May
Ogata AF, Cheng CA, Desjardins M, et al. Circulating SARS-CoV-2 Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin Inf Dis May 20, 2021, ciab465, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab465/6279075
In this study, 11/13 participants exhibited S1 antigen in plasma as early as day one after the first vaccine injection, while nucleocapsid concentrations were insignificant in all participants, confirming that the detected S1 originates from vaccination and not natural infection. The presence of S1 was likely due to the nature of the encoded mRNA-1273 spike protein, which contains a cleavable S1-S2 site and enables release of S1 from the spike trimer2. It is hypothesized that release of S1 protein could result from cleavage via mammalian cell proteases or circulating proteases.
21 May
White EM, Yang X, Blackman C, et al. Incident SARS-CoV-2 Infection among mRNA-Vaccinated and Unvaccinated Nursing Home Residents. NEJM May 19, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2104849?query=featured_coronavirus
This large study on 18,242 residents shows real-world effectiveness of the mRNA vaccines in reducing the incidence of asymptomatic and symptomatic SARS-CoV-2 infections in a vulnerable population in 280 nursing homes.
19 May
Roeker LE, Knorr DA, Thompson MC. et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia May 13, 2021. https://www.nature.com/articles/s41375-021-01270-w
Only half of fully vaccinated patients (two doses of mRNA vaccines) with CLL develop detectable anti-SARS-CoV-2 S1/S2 antibodies. There was a significant difference between rates of detectable anti-SARS-CoV-2 S1/S2 antibodies between treatment-naïve patients (17/18, 94%) and those who had received CLL directed therapy (6/26, 23%).
18 May
Today we focus on vaccines. There is plenty of good news to share: mRNA vaccines work in real-life, in the US, in Israel, and even after the first shot. These vaccines may offer some protection against the South African variant. However, switching between vaccines (first dose vs second) may also be a reasonable option and although a significant loss in protection from SARS-CoV-2 infection will occur over the first year, protection from severe disease should be largely retained.
Pilishvili T, Fleming-Dutra KE, Farrar JL, et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel — 33 U.S. Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 14 May 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e2.htm?s_cid=mm7020e2_w
This large US multisite test-negative design vaccine effectiveness study among HCP found a single dose of Pfizer-BioNTech or Moderna vaccines to be 82% effective against symptomatic COVID-19 and 2 doses to be 94% effective.
Chodick G, Tene L, Rotem RS, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clinical Infectious Diseases May 17, 2021, ciab438, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab438/6276888
Real life data from Israel. Incidence rate of a SARS-CoV-2 infection between 7 to 27 days after the second dose (the protection period) was compared to days 1 to 7 after the first dose, where no protection by the vaccine is assumed (the reference period). In 1.1 million vaccinated persons, vaccine effectiveness in preventing infection was 90% and 94% against COVID-19. Look at the beautiful Figure 1.
Spencer AJ, McKay PF, Belij-Rammerstorfer S. et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat commun May 17, 2021. 12, 2893. https://www.nature.com/articles/s41467-021-23173-1
Switching vaccines (first dose vs second) may induce strong immune responses. In mice, antibody responses were high with two-dose heterologous vaccination (first shot with mRNA, second with AstraZeneca, or vice versa) regimens. Neutralizing titers were at least comparable or higher than the titers measured after homologous prime boost vaccination with viral vectors. Importantly, the cellular immune response after a heterologous regimen was dominated by cytotoxic T cells and Th1+ CD4 T cells, which is superior to the response induced in homologous vaccination regimens. Several trials in humans are underway (https://comcovstudy.org.uk/home).
Bailly B, Guilpain L, Bouiller K, et al. BNT162b2 mRNA vaccination did not prevent an outbreak of SARS COV-2 variant 501Y.V2 in an elderly nursing home but reduced transmission and disease severity. Clinical Infectious Diseases, 16 May 2021. ciab446, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab446/6276392
An outbreak of SARS-CoV-2 1.351 (the South African variant) in a nursing home in Jura, in eastern France. 5/5 non-vaccinated residents versus 13/26 of those vaccinated with BNT162b2 were infected. Two of 13 vaccinated versus 4 of 5 non-vaccinated residents presented severe disease.
Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med May 17, 2021. https://www.nature.com/articles/s41591-021-01377-8
Using data from several large vaccine trials (because of the different assays used, neutralization titers were normalized to the mean convalescent titer using the same assay in the same study), the authors estimate the neutralization level for 50% protection against detectable SARS-CoV-2 infection to be 20% of the mean convalescent level. The estimated neutralization level required for 50% protection from severe infection was significantly lower (3%). Modeling of the decay of the neutralization titer over the first 250 d after immunization predicts that a significant loss in protection from SARS-CoV-2 infection will occur, although protection from severe disease should be largely retained.
17 May
Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years — United States, May 2021. MMWR Morb Mortal Wkly Rep. ePub: 14 May 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e1.htm?s_cid=mm7020e1_w#suggestedcitation
On May 10, 2021, the Food and Drug Administration expanded Emergency Use Authorization for the Pfizer-BioNTech COVID-19 vaccine to include adolescents aged 12–15 years. Read why here.
Wu K, Choi A, Koch M, et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. doi: https://doi.org/10.1101/2021.05.05.21256716
Not yet peer reviewed, but important, good news. In total, 40 people who had already been fully vaccinated with mRNA-1273, received a third shot 6 months later. A booster dose of mRNA-1273.351, Moderna’s variant-matched vaccine, achieved higher neutralizing antibody titers against the B.1.351 variant (the “immune escape” variant from South Africa) than a booster dose of mRNA-1273. However, the best news is that the titers were high enough to kill B.1.351 even in those who received the conventional vaccine.
15 May
Romero-Brufau S, Chopra C, Ryu A, et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ May 12, 2021; 373 doi: https://www.bmj.com/content/373/bmj.n1087
This simulation modeling study suggests that a delayed second dose vaccination strategy, at least for people aged under 65, could result in reduced cumulative mortality under certain conditions.
Liu Y, Liu J, Xia H, et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. NEJM May 12, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2106083?query=featured_home
The newly emerged B.1.526, B.1.429, and B.1.1.7+E484K variants remained susceptible to BNT162b2-elicited immune effector (neutralizing antibody).
14 May
Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ May 13, 2021; 373. https://www.bmj.com/content/373/bmj.n1088
Early real-life data in older people (older than 70 years) from England: with BNT162b2, vaccine effectiveness reached 61% from 28 to 34 days after vaccination, then plateaued. With ChAdOx1-S, effects were seen from 14 to 20 days after vaccination, reaching an effectiveness of 60% from 28 to 34 days, increasing to 73% (27% to 90%) from day 35 onwards.
13 May
Johnston MS, Galan A, Watsky KL, et al. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine A Case Series. JAMA Dermatol May 12, 2021. https://jamanetwork.com/journals/jamadermatology/fullarticle/2779643?resultClick=1
The “COVID vaccine arm”: Delayed localized cutaneous reactions, developing in 16 patients (13 women), a median (range) of 7 (2-12) days after receiving the Moderna COVID-19 vaccine. Reactions occurred near the injection site and were benign and self-limited. These reactions are not a contraindication to subsequent vaccination.
9 May
Angel Y, Spitzer A, Henig O, et al. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA May 6, 2021. https://jamanetwork.com/journals/jama/fullarticle/2779853
Among 6710 health care workers in Tel Aviv, 757 (11.3%) were not vaccinated. Symptomatic SARS-CoV-2 infection occurred in 8 fully vaccinated and 38 unvaccinated health care workers (adjusted IRR, 0.03). The corresponding numbers for asymptomatic SARS-CoV-2 infections were 19 and 17, respectively (adjusted IRR, 0.14). Results were qualitatively unchanged by the propensity score sensitivity analysis.
Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ May 5, 2021; 373. https://www.bmj.com/content/373/bmj.n1114
Among 281,264 people who received ChAdOx1-S, 11 and 2.5 excess events per 100,000 vaccinations were seen for venous thromboembolic events and cerebral venous thrombosis, respectively.
8 May
Boyarsky NJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA May 5, 2021. https://jamanetwork.com/journals/jama/fullarticle/2779852?resultClick=1
At a median of 29 days after dose 2, antibody was detectable in 357/658 organ recipients (54%). Poor humoral response was persistently associated with use of anti-metabolite immunosuppression.
7 May
Shiinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. NEJM May 5, 2021, https://www.nejm.org/doi/full/10.1056/NEJMoa2103055?query=featured_home
Early findings on the efficacy of a randomized, placebo-controlled, Phase IIa/b trial of Novavax’ nanoparticle vaccine NVX-CoV2373. The trial was conducted in 4347 predominantly young and healthy people in South Africa during a period of predominant circulation of the B.1.351 variant. Vaccine efficacy among HIV-negative participants was 60% (B.1.351: 51%). Severe COVID-19 was rare. Of note, previous infection with wild type viruses did not appear to reduce the risk of COVID-19 due to subsequent infection with B.1.351 variants among placebo recipients during the first 2 months of follow-up.
Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet May 05, 2021. DOI:https://doi.org/10.1016/S0140-6736(21)00947-8
By April 3, 2021, 4.7 M people aged 16 years and older were fully vaccinated with two doses of BNT162b2 in Israel. Vaccine effectiveness at 7 days or longer after the second dose was 95% against SARS-CoV-2 infection, 97% against symptomatic COVID-19, 97% against hospitalization, and 98% against severe or critical disease. In all age groups, as vaccine coverage increased, the incidence of SARS-CoV-2 outcomes declined. Estimated prevalence of the B.1.1.7 variant was 95%.
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. NEJM May 5, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2104974?query=featured_home
Following a mass immunization campaign with BNT162b2 in Qatar, as of March 31, 2021, a total of 385,853 persons had received at least one vaccine dose and 265,410 had completed both doses. The estimated effectiveness against any documented infection with the B.1.1.7 variant was 89.5% at 14 days or more after the second dose. The effectiveness against any documented infection with the B.1.351 variant was 75%. Of note, effectiveness against severe, critical, or fatal disease (with the B.1.1.7 and B.1.351 variants being predominant in Qatar) was very high, at 97%.
Thompson CN, Hughes S, Ngai S, et al. Rapid Emergence and Epidemiologic Characteristics of the SARS-CoV-2 B.1.526 Variant — New York City, New York, January 1–April 5, 2021. MMWR Morb Mortal Wkly Rep. ePub: 5 May 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7019e1.htm?s_cid=mm7019e1_w#suggestedcitation
The “New York variant”: although the SARS-CoV-2 B.1.526 variant emerged rapidly in NYC, early evidence suggests that this variant, even with the E484K mutation (found in 40%), does not lead to more severe disease and is not associated with increased risk for breakthrough infection or re-infection compared with other sequenced SARS-CoV-2 viruses.
Martin Webb L, Matzinger S, Grano C, et al. Identification of and Surveillance for the SARS-CoV-2 Variants B.1.427 and B.1.429 — Colorado, January–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 5 May 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7019e2.htm?s_cid=mm7019e2_w#suggestedcitation
The arrival of the two “California” variants B.1.427/B.1.429 in Colorado: the proportion of sequenced specimens increased from 3%–4% in late January to 20%–22% in early March. Some (very) preliminary data indicate that B.1.427/B.1.429 might more frequently cause discernible and severe illness than do other circulating lineages.
5 May
Fabiani M, Ramigni M, Gobbetto V, et al. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Eurosurveillance May 3, 2021. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.17.2100420
In this study on 6423 HCW from northern Italy, vaccine effectiveness (VE) was high. VE in preventing symptomatic infections during the time intervals 1–14 days and 15–28 days from administration of the first dose were 40% (95% CI: 9–60) and 86% (95% CI: 33–97), respectively.
4 May
Solforosi L, Kuipers H, Jongeneelen M, et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J Exp Med April 28 2021. https://rupress.org/jem/article/218/7/e20202756/212032/Immunogenicity-and-efficacy-of-one-and-two-doses?searchresult=1
In rhesus macaques, spike protein–specific immune responses were better with two-doses of the Ad26.COV2.S vaccine. In humans, a two-dose regimen is currently evaluated in a Phase 3 study (ENSEMBLE 2).
3 May
Hause AM, Gee J, Johnson T, et al. Anxiety-Related Adverse Event Clusters After Janssen COVID-19 Vaccination — Five U.S. Mass Vaccination Sites, April 2021. MMWR Morb Mortal Wkly Rep. ePub: 30 April 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7018e3.htm?s_cid=mm7018e3_w#suggestedcitation
Anxiety-related events, including fainting, can occur immediately after vaccination with any vaccine and might be caused by anxiety about receiving an injection. The stress of an ongoing pandemic might increase anxiety: Reports of syncope (fainting) are approximately 164 times more common after the Janssen COVID-19 vaccination (8.2 per 100,000) than after influenza vaccination (0.05 per 100,000).
2 May
See I, Su JR, Lale A, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA April 30, 2021. https://jamanetwork.com/journals/jama/fullarticle/2779731?resultClick=1
Twelve US cases of cerebral venous sinus thrombosis (CVST) with thrombocytopenia following vaccination with Ad26.COV2.S, the COVID-19 vaccine produced by Janssen/J&J. All were women, younger than 60 years, and had symptom onset ranging from 6 to 15 days after vaccination that required hospitalization.
30 April
Tenforde MW, Olson SM, Self WH. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021. MMWR April 28. https://www.cdc.gov/mmwr/volumes/70/wr/mm7018e1.htm?s_cid=mm7018e1_w
In a multi-state network of US hospitals during January–March 2021, receipt of Pfizer/BioNTech or Moderna COVID-19 vaccines was 94% effective against COVID-19 hospitalization among fully vaccinated adults and 64% effective among partially vaccinated adults aged ≥ 65 years. There was no significant effect for receiving the first dose of a 2-dose COVID-19 vaccine series within 14 days prior to illness onset.
29 April
MacNeil JR, Su JR, Broder KR, et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients — United States, April 2021. MMWR Morb Mortal Wkly Rep. ePub: 27 April 2021. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e4.htm?s_cid=mm7017e4_w
On April 23, the Advisory Committee on Immunization Practices concluded that the benefits of resuming Janssen’s vaccination in persons aged ≥ 18 years outweigh the risks. Up to now, there are 15 cases of cerebral venous sinus thrombosis (CVST), mostly among women aged 18−49 years. Two occurred among women aged ≥ 50 years; no cases post-authorization were reported among men. Reporting rates were 7.0 cases per million doses administered to women aged 18−49 years and 0.9 per million to women aged ≥ 50 years.
Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Inf Dis April 27, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00224-3/fulltext
Between Dec 8, and March 10, 2021, 627,383 individuals reported being vaccinated: 282,103 received one dose of BNT162b2, of whom 28,207 received a second dose, and 345,280 received one dose of ChAdOx1 nCoV-19. Systemic side effects were reported by 13.5%, 22.0%, and by 33.7%, respectively. Local side effects were reported by 71.9%, 68.5% and 58.7%. Systemic side effects were more common (1.6 times after the first dose of ChAdOx1 nCoV-19 and 2.9 times after the first dose of BNT162b2) among individuals with previous SARS-CoV-2 infection.
Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Inf Dis April 27, 2021. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(21)00213-8/fulltext
Better not wait too long with the second shot in cancer patients? In this prospective observational study of 151 patients with solid and hematological cancers, one 30 μg dose of the BNT162b2 vaccine yielded poor efficacy, as measured by seroconversion rates, viral neutralization capacity, and T cell responses, at 3 weeks and 5 weeks following the first inoculum. Immunogenicity increased significantly in patients with solid cancer within 2 weeks of a vaccine boost at day 21 after the first dose.
25 April
Shimabukuro TT, Kim SY, Myers TR. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. NEJM April 22, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104983?query=featured_home
Data on more than 35,000 pregnant women receiving mRNA vaccines (BioNTech or Moderna) indicate no safety signals. However, as this was a participant-reported surveillance system, more data is needed.
Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet April 23, 2021. https://www.thelancet.com/action/showPdf?pii=S0140-6736%2821%2900677-2
Paper of the day! Between Dec 8, 2020, and Feb 22, 2021, a total of 1,331,993 people were vaccinated in Scotland. The first dose of the BNT162b2 mRNA vaccine was associated with a vaccine effect of 91% (95% CI 85–94) for reduced COVID-19 hospital admission at 28–34 days post-vaccination. Vaccine effect for the ChAdOx1 vaccine was 88% (95% CI: 75–94). Results of combined vaccine effects against hospital admission due to COVID-19 were similar the analysis was restricted to those aged 80 years and older (83%, 95% CI: 72–89).
23 April
Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. NEJM Apr 22, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2101544?query=featured_home
Paper of the day. In this large Phase III study, Johnson & Johnson’s Ad26.COV2.S vaccine protected 67% (66%) against moderate to severe/critical COVID-19 with onset at least 14 (28) days after administration. Vaccine efficacy was higher against severe/critical disease (85% for onset at ≥ 28 days). Despite 86 of 91 cases (94.5%) in South Africa with sequenced virus having the 20H/501Y.V2 variant, vaccine efficacy was 82% against severe/critical COVID-19 with onset at least 28 days after administration.
Chappell KJ, Mordant FL, Li Z, et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Inf Dis April 19, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00200-0/fulltext
This first-in-human trial shows that a subunit vaccine comprising mammalian cell culture-derived, MF59-adjuvanted, molecular clamp-stabilized recombinant spike protein elicits strong immune responses with a promising safety profile. However, as the glycoprotein 41 peptide present in the clamp created HIV cross-reactivity, this specific vaccine design will not be progressed further.
Cavanaugh AM, Fortier S, Lewis P, et al. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program — Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. ePub: 21 April 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e2.htm?s_cid=mm7017e2_w#suggestedcitation
In a COVID-19 outbreak at a Kentucky skilled nursing facility involving a newly introduced variant (characterized by E484K and other mutations within the spike protein) to the region, 26/83 residents (18/75 fully vaccinated with BioNTech) and 20/116 HCP (4/61 vaccinated) became infected. Vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective among HCP. Three residents died, two of whom were unvaccinated (vaccine effectiveness 94%).
Teran RA, Walblay KA, Shane EL, et al. Postvaccination SARS-CoV-2 Infections Among Skilled Nursing Facility Residents and Staff Members — Chicago, Illinois, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 21 April 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e1.htm?s_cid=mm7017e1_w#suggestedcitation
During the investigation period, an estimated 7931 skilled nursing facility residents and 6834 staff members received two doses of the COVID-19 vaccine. Among 12 possible breakthrough SARS-CoV-2 infections among fully vaccinated residents ≥ 14 days after their second dose of COVID-19 vaccine, 8 were asymptomatic.
Hacisuleyman E, Hale H, Saito Y, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. NEJM April 21, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2105000?query=featured_home
Two breakthrough cases. Despite evidence of vaccine efficacy in both women, symptoms of COVID-19 developed, and they tested PCR positive 19 and 36 days after the second MODERNA shot. Rapid identification of sequence variants by targeted PCR amplification showed that neither sequence precisely fit any known clade.
22 April
Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Inf Dis April 19, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00147-X/fulltext
Recombinant protein vaccines offer the advantages of fewer potential safety concerns and lower production costs than other traditional (eg, attenuated or inactivated) vaccines. However, they often require the use of an adjuvant to enhance the magnitude and quality of the immune response. In this first-in-human evaluation, CoV2 preS dTM (a stabilized pre-fusion spike protein vaccine produced in a baculovirus expression system) had suboptimal immunogenicity and greater than expected reactogenicity, requiring optimization of the antigen formulation.
21 April
Rossman H, Shilo S, Meir T, et al. COVID-19 dynamics after a national immunization program in Israel. Nat Med April 19, 2021. https://www.nature.com/articles/s41591-021-01337-2
By no doubt the paper of the day, analyzing the early effect of the national vaccination campaign in Israel on the pandemic dynamics. A little over 2 months after the initiation of the campaign, with 85% of individuals older than 60 years already vaccinated with two doses (24 February 2021), there was an approximately 77% drop in cases, a 45% drop in positive test percentage, and a 68% drop in hospitalizations compared to peak values. Larger and earlier decrease in COVID-19 cases and hospitalization was observed in individuals older than 60 years, followed by younger age groups, by the order of vaccination prioritization. This pattern was not observed in the previous lockdown and was more pronounced in early-vaccinated cities.
20 April
Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood April 16, 2021. https://ashpublications.org/blood/article/doi/10.1182/blood.2021011568/475742/Efficacy-of-the-BNT162b2-mRNA-COVID-19-Vaccine-in?searchresult=1
Antibody response to BNT162b2 mRNA COVID-19 vaccine in CLL patients is markedly impaired and affected by disease activity and treatment. In a total of 167 patients with CLL who received two doses, the antibody response rate was only 39.5%. In patients treated with either Bruton’s tyrosine kinase inhibitors or venetoclax ± anti-CD20 antibody, responses were particularly low (16% and 14%).
19 April
Blain H, Tuaillon E, Gamon L, et al. Spike Antibody Levels of Nursing Home Residents With or Without Prior COVID-19 3 Weeks After a Single BNT162b2 Vaccine Dose. JAMA April 15, 2021. https://jamanetwork.com/journals/jama/fullarticle/2778926?resultClick=1
One shot may be enough in people with previous COVID-19. What had already been described in HCW (https://jamanetwork.com/journals/jama/fullarticle/2777171?resultClick=1) may also be true for nursing home residents: all 36 residents (100%) with prior COVID-19 were seropositive for S protein IgG after one mRNA vaccine dose vs only 29 of 60 residents (49.2%) without prior COVID-19.
18 April
Scully M, Sing D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19. NEJM April 16, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2105385
This case series of 23 patients from London who presented with thrombosis and thrombocytopenia 6 to 24 days after receiving the first dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca). All patients had D-dimer levels at presentation much higher than what would be expected in patients with acute venous thromboembolism.
Muir KL, Kallam A, Koepsell SA, et al. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. NEJM April 14, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2105869
First case seen with the Ad26.COV2.S vaccine from Johnson & Johnson. A 48-year-old White woman presenting with extensive thrombosis associated with severe thrombocytopenia and disseminated intravascular coagulation. The patient remained critically ill at the time of this report.
Sadoff J, Davis K, Douoguih M. Thrombocytopenia after Ad26.COV2.S Vaccination — Response from the Manufacturer. NEJM April 16, 2021, https://www.nejm.org/doi/full/10.1056/NEJMc2106075
Response from Janssen: Their ongoing safety surveillance received reports of six cases of CVST with thrombocytopenia occurring 7 to 14 days after vaccination, including the above case. These cases were reported among more than 7.2 million persons who had been vaccinated with Ad26.COV2.S globally as of April 14. Janssen argues (and surely hopes) that their numbers are lower, due to substantial vector and spike differences between their vaccine and Astra Zeneca’s. Their vaccine uses a human Ad26–based vector (vs the chimpanzee one for ChAdOx1 nCoV-19) with different host cell receptors and is likely to have different phylogenetic and biologic characteristics. Let’s hope.
Ledford H. COVID vaccines and blood clots: five key questions. Nature News April 16, 2021. https://www.nature.com/articles/d41586-021-00998-w
Heidi Ledford summarizes the key questions (but has no definite answers): What could the connection be between blood clots and vaccines? Are other COVID-19 vaccines linked to blood clotting disorders? How rare are they and are certain groups of people more at risk? What impact will fears over potential side effects have on global vaccination efforts? Answers needed.
16 April
Anichini G, Terrosi C Gandolfo C, et al. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. NEJM April 14, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2103825?query=featured_coronavirus
This study indicates a significantly lower neutralizing antibody titer after administration of a second dose of vaccine in previously uninfected patients than the titer after only a single dose of vaccine in previously infected participants.
11 April
Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. NEJM April 9, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104840?query=featured_home
A case series of 11 patients (9 women) from Germany and Austria in whom thrombosis or thrombocytopenia developed after vaccination with ChAdOx1 nCov-19. All had moderate-to-severe thrombocytopenia and thrombotic complications at unusual sites beginning 5-16 days after first vaccination. All had platelet-activating antibodies directed against platelet factor 4 (PF4)–heparin.
Schultz NH, Sørvoll ICH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. NEJM April 9, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2104882?query=featured_home
Another five patients (four women) from Norway. Same findings.
10 April
Van Praet JT, Vandecasteele S, de Roo A, et al. Humoral and cellular immunogenicity of the BNT162b2 mRNA Covid-19 Vaccine in nursing home residents. Clin Inf Dis, ciab300, April 7, 2021. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab300/6213866?searchresult=1
Four weeks after the first dose, the humoral and cellular immunogenicity of the BNT162b2 mRNA vaccine (BioNTech/Pfizer) was suboptimal in COVID-19-naïve nursing home residents in comparison to COVID-19-naïve healthcare workers.
Moyo-Gwete T, Madzivhandila M, Makhado Z, et al. Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351). NEJM April 7, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2104192?query=featured_home
Infection with B.1.351 (South Africa) elicits robust neutralizing antibody responses against P.1 (Brazil) and the original variants, which indicates high levels of cross-reactivity. Vaccines built on the spike protein of B.1.351 may be promising candidates for the elicitation of cross-reactive responses.
Lustig Y, Nemet I, Kliker L, et al. Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2. NEJM April 7, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2104036?query=featured_home
In six HCW who had been infected with the original virus, one shot of BNT162b2 (BioNTech/Pfizer) induced robust neutralizing antibody responses against all variants of concern, including B.1.351 from South Africa.
9 April
Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 Apr 6. PubMed: https://pubmed.gov/33822494. https://www.nejm.org/doi/10.1056/NEJMc2103916
In 33 healthy adult participants in an ongoing Phase I trial, antibodies that were elicited by mRNA-1273 (Moderna) persisted through 6 months after the second dose, as detected by three distinct serologic assays.
5 April
Rottenstreich A, Zarbiv G, Oiknine-Dijan E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clinical Infectious Diseases 03 April 2021, ciab266. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab266/6209876?searchresult=1
In 20 pregnant women, two doses of BNT162b2 mRNA vaccine (median time between the second dose until delivery was 11 days) induced an adequate maternal serologic response that had the potential to provide neonatal protection through transplacental transfer of vaccine-stimulated maternally-derived antibodies.
4 April
Cobey S, Larremore DB, Grad YH. et al. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat Rev Immunol April 2, 2021. https://www.nature.com/articles/s41577-021-00544-9
See title. Marc Lipsitch and colleagues argue that as long as vaccination provides some protection against escape variants, the corresponding reduction in prevalence and incidence should reduce the rate at which new variants are generated and the speed of adaptation.
Malhotra HS, Gupta P, Prabhu V. COVID-19 vaccination-associated myelitis. QJM 31 March 2021, hcab069, https://academic.oup.com/qjmed/advance-article/doi/10.1093/qjmed/hcab069/6206408?searchresult=1
A rare case of myelitis, seen with the ChAdOX1 nCoV-19 vaccine. On the 8th day post-vaccination, this 38-yr-old man presented with abnormal sensations in both lower limbs.
2 April
Jentsch PC, Anand M, Bauch CT. Prioritising COVID-19 vaccination in changing social and epidemiological landscapes: a mathematical modelling study. Lancet Inf Dis March 31, 2021. https://www.thelancet.com/action/showPdf?pii=S1473-3099%2821%2900057-8
The oldest-first strategy is not always the best option. This modelling study shows that in populations in which SARS-CoV-2 seropositivity is high, a contact-based strategy allocating vaccines according to the relative role played by different age groups in transmission may be more effective that targeting vulnerable groups.
1 April
Paper of the Day
Emary KR, Gulobchik T, Aley PK. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet March 30, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00628-0/fulltext
This post-hoc analysis of the efficacy of the adenoviral vector vaccine, ChAdOx1 nCoV-19 (AZD1222), revealed that laboratory virus neutralization activity by vaccine-induced antibodies was lower against B.1.1.7. However, clinical vaccine efficacy against symptomatic NAAT positive infection was good, with 70% (95% CI 44–85) for B.1.1.7 and 82% (68–89) for other lineages.
31 March
Thompson MG, Burgess JL, Naleway AL, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 29 March 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7013e3.htm
In this prospective cohort of 3950 health care personnel, first responders, and other essential and frontline workers who completed weekly SARS-CoV-2 testing for 13 consecutive weeks, mRNA vaccine effectiveness of full immunization (≥ 14 days after second dose) was 90% against SARS-CoV-2 infections regardless of symptom status; vaccine effectiveness of partial immunization (≥ 14 days after first dose but before second dose) was 80%.
28 March
ANSM 20210326. Point de situation sur la surveillance des vaccins contre la COVID-19 – Période du 12/03/2021 au 18/03/2021. Agence nationale pour la sécurité du médicament et des produits de Santé 2021, published 26 March 2021. Full text: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-12-03-2021-au-18-03-2021
On 26 March, the French National Medicine Safety Agency (Agence nationale pour la sécurité du médicament et des produits de Santé – ANSM) declared that there is a risk of atypical thrombosis associated with the AstraZeneca vaccine. The Agency reports 9 cases of big vein thromboses that are atypical by their location (mostly cerebral, but also digestive) and associated with thrombocytopenia and coagulation disorders. With some 1,430,000 injections of the AstraZeneca vaccine as of 18 March 2021, this is one case per 158,000 injections. These cases occurred within a median time of 8.5 days after vaccination in persons without particular risk factors (7 patients under 55 years of age, 2 more than 55 years). Between 12 and 18 March, two deaths were reported, including that of a medical student who died several days after being vaccinated. In the coming months of relative vaccine abundance, the AstraZeneca vaccine does not have a good star.
McEllistrem MC, Clancy CJ, Buehrle DJ, et al. Single dose of a mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clin Infect Dis 2021, published 26 March. Full text: https://doi.org/10.1093/cid/ciab263
In nursing home residents with asymptomatic COVID-19 diagnosed through twice-weekly surveillance testing, single dose BNT162b2 vaccination (Pfizer-BioNTech) was associated with -2.4 mean log10 lower nasopharyngeal viral load than that detected in absence of vaccination (p = 0.004).
Irwin A. What it will take to vaccinate the world against COVID-19. Nature 2021, published 25 March. Full text: https://www.nature.com/articles/d41586-021-00727-3
Within just a few months, pharmaceutical firms have produced hundreds of millions of doses of COVID-19 vaccine. But the world needs billions — and as fast as possible. This situation is fuelling a campaign to temporarily waive intellectual-property rights so that manufacturers in poorer countries can make the vaccines more quickly themselves.
27 March
Hughes MM, Wang A, Grossman MK, et al. County-Level COVID-19 Vaccination Coverage and Social Vulnerability — United States, December 14, 2020–March 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:431–436. Full text: http://dx.doi.org/10.15585/mmwr.mm7012e1
In the first 2.5 months of the US vaccination program, high social vulnerability counties had lower COVID-19 vaccination coverage than did low social vulnerability counties. Although vaccination coverage estimates by county-level social vulnerability varied widely among states, disparities in vaccination coverage were observed in the majority of states.
26 March
Stamatatos L, Czartoski J, Wan YH, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, published 25 March. Full text: https://science.sciencemag.org/content/early/2021/03/24/science.abg9175
The preprint we presented on 10 February now published in Science. The study highlights the importance of vaccinating both uninfected and previously infected persons to elicit cross-variant neutralizing antibodies.
Desmond a, Offit PA. On the Shoulders of Giants — From Jenner’s Cowpox to mRNA Covid Vaccines. N Engl J Med 2021; 384:1081-1083. Full text: https://doi.org/10.1056/NEJMp2034334
mRNA vaccines will change the course of the COVID-19 pandemic and the impact of other infectious diseases. Their development goes back to 2008 when Katalin Karikó, Drew Weissman, and colleagues modified messenger RNA (mRNA) using nucleoside analogues. These modifications stabilized the molecule and eliminated its capacity for inducing innate immunity, thereby making mRNA a vaccination tool (Karikó K 2008). A short vaccine history for the weekend.
Abdool Karim SS, de Oliveira T. New SARS-CoV-2 Variants — Clinical, Public Health, and Vaccine Implications. N Engl J Med 2021, published 24 March. Full text: https://doi.org/10.1056/NEJMc2100362
An excellent summary of our Variants chapter by @ProfAbdoolKarim and @Tuliodna.
Bradley T, Grundberg E, Selvarangan R, et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med 2021, 2021, published 23 March. https://www.nejm.org/doi/full/10.1056/NEJMc2102051
Three weeks after a single vaccination, persons with recent SARS-CoV-2 infection or seropositive status had higher levels of antibody to four SARS-CoV-2 antigens and higher levels of antibodies with neutralizing characteristics than did those with no history of infection.
25 March
Keehner J, Pfeffer MA, Longhurst CA, et al. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N Engl J Med 2021, 2021, published 23 March. https://www.nejm.org/doi/full/10.1056/NEJMc2101927
COVID-19 infection after complete vaccination? Possible, but rare. At the University of California, San Diego (UCSD) and the University of California, Los Angeles (UCLA) health systems, 379 persons (/36,659 who received the first dose, and /28,184 of whom received the second dose) tested positive for SARS-CoV-2 at least 1 day after vaccination, the majority (71%) of whom tested positive within the first 2 weeks after the first dose. After receiving both vaccinations, 37 health care workers tested positive; of these workers, 22 had positive test results 1 to 7 days after the second dose. Only 8 health care workers tested positive 8 to 14 days after the second vaccination, and 7 tested positive 15 or more days after the second vaccination.
Daniel W, Nivet M, Warner J, Podolsky KD. Early Evidence of the Effect of SARS-CoV-2 Vaccine at One Medical Center. N Engl J Med 2021, 2021, published 23 March. https://www.nejm.org/doi/full/10.1056/NEJMc2102153
Vaccine efficacy among employees of a medical center. Daniel et al. report 234 SARS-CoV-2 infections in 8969 non-vaccinated employees, 112 in 6144 partially vaccinated employees, and 4 among 8121 fully vaccinated employees, p < 0.01 for all pairwise comparisons. Next problem: vaccine hesitancy. Only 78% of employees were vaccinated by March 5.
23 March
Khoury DS, Cromer D, Reynaldi A, et al. What level of neutralising antibody protects from COVID-19? medRxiv 2021, posted 11 March. Full-text: https://doi.org/10.1101/2021.03.09.21252641
Predictive models of immune protection are useful to identify immune correlates of protection to assist in the future deployment of vaccines. Here, the authors modelled the relationship between in vitro neutralisation levels and observed protection from SARS-CoV-2 infection using data from seven current vaccines as well as convalescent cohorts. They report that neutralisation level is highly predictive of immune protection. Attention: this is a pre-print, so don’t take the data for granted. However, in the future, with vaccines abundantly available, we will use figures such as Figure 1 to decide whether to prefer product A over product B.
Tauzin A, Nayrac M, Benlarbi M, et al. A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses. bioRxiv 2021, posted 18 March. Full-text: https://doi.org/10.1101/2021.03.18.435972
No neutralising activity in SARS-CoV-2 naïve individuals three weeks after a single dose of the Pfizer-BioNTech vaccine. However, the authors detected strong anti-receptor binding domain (RBD) and spike antibodies with Fc-mediated effector functions and cellular responses dominated by the CD4+ T cell component. These data provide support to spacing the doses of two-vaccine regimens to vaccinate a larger pool of the population in the context of vaccine scarcity against SARS-CoV-2.
21 March
Hassan AO, Feldmann F, Zhao H, et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep Med March 17, 2021. https://www.cell.com/action/showPdf?pii=S2666-3791%2821%2900046-X
The future? An intranasally-administered chimpanzee adenovirus-vectored vaccine encoding a pre-fusion stabilized spike (S) protein (ChAd-SARS-CoV-2-S) worked well in macaques. A single intranasal dose induced neutralizing antibodies and T cell responses and limited or prevented infection in the upper and lower respiratory tract after a SARS-CoV-2 challenge.
20 March
Moore S, Hill EM, Tildesley MJ, et al. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Inf Dis March 18, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00143-2/fulltext
Vaccination alone is not enough. This modeling study from UK indicates that in the absence of non-pharmaceutical interventions (NPIs), even with the most optimistic assumption that the vaccine will prevent 85% of infections, R is estimated to be 1.58 (95% CI 1.36–1.84) once all eligible adults have been offered both doses of the vaccine. Under the default uptake scenario, removal of all NPIs once the vaccination program is complete is predicted to lead to 21,400 deaths due to COVID-19 for a vaccine that prevents 85% of infections, although this number increases to 96,700 deaths if the vaccine only prevents 60% of infections.
18 March
Mahdi SA, Baillie C, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. NEJM, March 16, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2102214
No protection from mild-to-moderate COVID-19 with AstraZeneca in this large RCT on young (median age 30 years), HIV-negative patients in South Africa. Infections were seen in 23 of 717 placebo recipients (3.2%) and in 19 of 750 vaccine recipients (2.5%), for an efficacy of 21.9% (95% CI, −49.9 to 59.8). Main caveat: as there were no cases of hospitalization in the study, it remains unclear whether ChAdOx1 nCov-19 may protect against severe infection with the B.1.351 variant.
Krutikov M, Hayward A, Shallcrosse L. Spread of a Variant SARS-CoV-2 in Long-Term Care Facilities in England March 16, 2021. NEJM March 16, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2035906
Rapid spread in staff and residents of long-term care facilities in the UK. Within a few weeks (between November 16 and December 13), the proportion of positive samples with S gene target failure (a proxy for B.1.1.7) increased from 70 of 582 (12.0%) to 491 of 813 (60.4%). This increase was associated with a decrease in median Ct values (from 27 to 21).
17 March
Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA March 15, 2021. https://jamanetwork.com/journals/jama/fullarticle/2777685?resultClick=1
In this multicenter study on 436 solid organ transplant recipients taking different immunosuppressive agents, only 76 (17%) had detectable antibodies (anti-S1 or anti–receptor-binding domain) at a median of 20 days after the first dose of vaccine. These results contrast with the robust early immunogenicity observed in mRNA vaccine trials.
Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks — Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. ePub: 15 March 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7011e3.htm
Retrospective study in a total of 463 residents of two skilled nursing facilities, estimating effectiveness of partial vaccination in preventing SARS-CoV-2 infection to be 63% (95% CI: 33%–79%).
14 March
Shah A, Gribben C, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. GitHub preprint, posted 12 March. Full-text: https://github.com/ChronicDiseaseEpi/hcw/blob/master/vaccine_manuscript.pdf
Household members of vaccinated healthcare workers have a lower risk for being infected with SARS-CoV-2 (rate per 100 person-years 9∙40 versus 5∙93; HR 0∙70, 95% CI: 0∙63 to 0∙78). This is the result of a Scottish cohort comprised of 194,362 household members (mean age 31∙1 ± 20∙9 years) and 144,525 healthcare workers (mean age 44∙4 ± 11∙4 years). As household members of healthcare workers can also be infected by other people, this 30% risk reduction is probably an underestimate of the effect vaccination will have on transmission of SARS-CoV-2.
Fischer R, van Doremalen N, Adney D, et al. ChAdOx1 nCoV-19 (AZD1222) protects against SARS-CoV-2 B.1.351 and B.1.1.7. bioRxiv 2021, posted 11 March. Full-text: https://doi.org/10.1101/2021.03.11.435000
Thinking of maybe not getting the vaccine because of the new variants? Think again! Here, Robert Fischer, Neeltje van Doremalen and colleagues show a lack of disease in Syrian hamsters vaccinated with the AstraZeneca vaccine when infected with B.1.1.7 or B.1.351 (first detected in the UK and South Africa, respectively). The authors observed a 9.5-fold reduction of virus-neutralizing antibody titer in vaccinated hamster sera against B.1.351 compared to B.1.1.7. Vaccinated hamsters challenged with B.1.1.7 or B.1.351 did not lose weight compared to control animals. Interesting data from a pre-print paper that need to be confirmed in clinical vaccine practice.
Public Health England 20210222. PHE monitoring of the early impact and effectiveness of COVID-19 vaccination in England. UK Government 2021, 22 February; accessed 5 March 2021. Full-text: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963532/COVID-19_vaccine_effectiveness_surveillance_report_February_2021_FINAL.pdf
Public Health England reports on 11,860 confirmed cases of COVID in those aged over 80 years. The Pfizer-BioNTech vaccine effectiveness was 57% (95% CI: 48-63%) from 28 days after the first dose of vaccination. In 8119 individuals aged over 80 years with a confirmed PCR positive test and followed for at least 21 days, the case fatality ratio was lower in cases vaccinated at least 14 days before onset than in unvaccinated cases. This would indicate that within vaccinated individuals who do become symptomatic the vaccine confers additional protection against death.
Ghebreyesus TA. A ‘me first’ approach to vaccination won’t defeat Covid. The Guardian 2021, published 5 March. Full-text: https://www.theguardian.com/commentisfree/2021/mar/05/vaccination-covid-vaccines-rich-nations
Of the 225m vaccines administered so far, most have been in a handful of rich nations. This has to change, for all our sakes, says Tedros Adhanom Ghebreyesus, director general of WHO. The normal rules of business that protect the profits of vaccine manufacturers will have to be set aside if that is what it takes to ensure everybody is immunized against SARS-CoV-2. See also Boseley S. A ‘me first’ approach to vaccination won’t defeat Covid. The Guardian 2021, published 5 March. Full-text: https://www.theguardian.com/world/2021/mar/05/covid-vaccines-who-chief-backs-patent-waiver-to-boost-production
Boseley S, Brooks L. UK will diverge from EU and US on approving tweaked Covid vaccines. The Guardian 2021, published 4 March. Full-text: https://www.theguardian.com/society/2021/mar/04/vaccines-tweaked-for-covid-variants-will-be-fast-tracked-safely-says-uk-regulator
The UK will adopt a different standard from Europe and the US when it considers approval for coronavirus vaccines that have been tweaked to deal with variants, the UK regulator has said.
13 March
Krammer F, Srivastava K, Alshammary H, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. March 10, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2101667
In 110 vaccinees, the antibody titers of those with pre-existing infection were 10 to 45 times higher than those of vaccinees without pre-existing immunity at the same time points after the first mRNA vaccine dose. The problem with all these studies: until we know whether this translates into similar protection, the one-shot strategy will not gain widespread acceptance. When we do know one day, this will largely not matter (there will be enough vaccine available) and will only be interesting for cost reasons.
Steinberg J, Thomas A, Iravavi A. 18Fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet March 08, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00464-5/fulltext
Julie Steinberg and colleagues from St. Louis describe an interesting case of a (self-resolving) systemic inflammatory response syndrome in a 65-year-old woman, starting one day after Moderna’s mRNA vaccine. PET/CT showed uptake in the fat stranding posterior to the right deltoid, moderately increased uptake within multiple right axillary lymph nodes and diffusely increased splenic uptake.
Fernández-Prada M, Rivero-Calle I, Calvache-González A, Martinón-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021;26(10):pii=2100193. https://doi.org/10.2807/1560-7917.ES.2021.26.10.2100193
María Fernández-Prada and colleagues report on 20 female HCWs with acute onset of a single supraclavicular lymphadenopathy coinciding with the ipsilateral intramuscular administration of an mRNA vaccine. Of note, 17 reported that the injection point had been unusually high. All lymphadenopathies had inflammatory symptoms (pain, swelling), were rounded and mobile, and all but one appeared in the first 24 h to 9 days after vaccine administration. All improved clinically, and 15 completely resolved between 5 and 16 days from onset.
12 March
Paper of the Day
Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA March 11, 2021. https://jamanetwork.com/journals/jama/fullarticle/2777598
The COVID-19 vaccine from Janssen (Johnson & Johnson) is the fourth vaccine recommended in the EU for preventing COVID-19. Ad26.COV2.S is a recombinant, replication-incompetent Ad26 vector encoding the full length and stabilized SARS-CoV-2 S protein derived from the first Wuhan strain. This phase I study (in 25 recipients from Boston) shows that a single immunization induced rapid binding and neutralization antibody responses as well as cellular immune responses, including induction of RBD-specific binding antibodies in 90% of vaccine recipients by day 8.
Vijayasingham L, Bischof E, Wolfe J. Sex-disaggregated data in COVID-19 vaccine trials. Lancet March 05, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00384-6/fulltext
Lavanya Vijayasingham and colleagues argue that sex factors, including sex-disaggregated analysis and reporting, are still neglected in COVID-19 trial data reporting.
11 March
Saad-Roy CM, Morris SE, Metcalf JE, et al. Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. Science 09 Mar 2021: eabg8663. https://science.sciencemag.org/content/early/2021/03/08/science.abg8663
Delaying the second dose? Maybe easier said than done. Chadi Saad-Roy and colleagues urge caution. According to their models, a vaccine strategy with a very long inter-dose period could lead to marginal short-term benefits at the cost of a higher infection burden in the long term and substantially more potential for viral evolution and the development of new variants. However, current uncertainties surrounding the strength and duration of adaptive immunity in response to natural infection or vaccination lead to very broad range of possible outcomes of various dosing regimens.
Capetti AF, Stangalini CA, Borgonovo F, et al. Impressive boosting of anti-S1/S2 IgG production in COVID-19-experienced patients after the first shot of the BNT162b2 mRNA COVID-19 Vaccine. Clinical Infectious Diseases 06 March 2021, ciab214, https://doi.org/10.1093/cid/ciab214
But is one shot enough after infection? The next study, comparing COVID-19 naïve people versus asymptomatic/pauci-symptomatic (A/P) people versus symptomatic/hospitalized (S/H) COVID-19 patients. Titers (logarithmic scale!) before and after the first dose of the BNT162b2 vaccine.
10 March
Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet March 08, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00070-0/fulltext
Inactivated vaccines have the advantage of being easily stored and shipped. BBV152 (COVAXIN) is a whole-virion inactivated SARS-CoV-2 vaccine adjuvanted with Algel-IMDG. An imidazoquinoline molecule (IMDG), a TLR7/8 agonist, is added to augment cell-mediated responses. According to the Phase I/II data from India presented here, BBV152 has shown the potential to provide durable humoral and cell-mediated immune responses (even against variants of concern). The Algel-IMDG formulation was selected for the ongoing Phase III efficacy trial, which involves 25,800 volunteers. BBV152 has received emergency use authorisation in India.
Rapaka RR, Hammershaimb EA, Neuzil KM. Are some COVID vaccines better than others? Interpreting and comparing estimates of efficacy in trials of COVID-19 vaccines. Clinical Infectious Diseases 06 March 2021, ciab213, https://doi.org/10.1093/cid/ciab213
Do you have colleagues who still “prefer” the BioNTech vaccine? Give him this viewpoint to read. Rekha Rapaka and colleagues from Maryland discuss the caveats of cross-trial comparisons. And why it matters how point estimates of efficacy were determined, in what epidemiologic setting, and against what endpoints.
Blumenthal KG, Robinson LB, Camargo Jr CA, et al. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA March 8, 2021; https://jamanetwork.com/journals/jama/fullarticle/2777417?resultClick=1
Of 64,900 vaccine recipients in Massachusetts, anaphylaxis was confirmed in 16 (0.025%). Of note, 15/16 were female, 10 had a prior history of allergies and 5 had a history of anaphylaxis. Mean time to anaphylaxis onset was 17 minutes (range, 1-120). All recovered.
9 March
Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021 Mar 4:1-2. PubMed: https://pubmed.gov/33661328. Full-text: https://doi.org/10.1007/s00259-021-05279-2
Lymphadenopathy is seen in some people after vaccination. In this case report of a cancer patient who underwent PET/CT 3 days after vaccination, nodal uptake was reactive in the axilla. Nice pictures.
7 March
Vasileiou E, Simpson CR, Robertson C, et al. Effectiveness of First Dose of COVID-19 Vaccines Against Hospital Admissions in Scotland: National Prospective Cohort Study of 5.4 Million People. Lancet Preprints 2021, posted 19 February. Full-text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3789264
A single dose of the Pfizer-BioNTech or AstraZeneca vaccine results in substantial reductions in the risk of COVID-19 related hospitalization. In a study from Scotland, the first vaccine dose protected well over 80% of vaccinees against COVID-19 related hospitalization at 28-34 days post-vaccination (Pfizer-BioNTech: 85%; AstraZeneca: 94%). Comparable results (81%) were seen in people aged ≥ 80 years (Vasileiou 2021).
Hall VJ, Foulkes S, Saei A, et al. Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study) Victoria Jane Hall. Lancet Preprints 2021, published 22 February. Full-text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3790399
These data from the SIREN (Sarscov2 Immunity and REinfection EvaluatioN) study suggest that the Pfizer-BioNTech vaccine effectively prevents both symptomatic and asymptomatic infection in working age adults. (The SIREN study is a prospective cohort study among staff working in publicly funded hospitals.) The vaccine was 72% effective (95% CI 58-86) 21 days after first dose and 86% effective (95% CI 76-97) seven days after two doses in the antibody negative cohort (Hall 2021).
5 March
Kimberly G. Blumenthal KG, Freeman EE, Saff RR. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. NEJM, March 3, 2021. DOI: 10.1056/NEJMc2102131. https://www.nejm.org/doi/full/10.1056/NEJMc2102131
Case series of 12 delayed large but varying local reactions to the mRNA-1273 vaccine, with a median onset on day 8 after the first dose. After the second shot, 6 had no recurrence, 3 had similar and 3 had recurrent reactions that were of a lower grade than those after the initial dose.
Kadire SR, Wachter RM, Lurie N. Delayed Second Dose versus Standard Regimen for Covid-19 Vaccination. N Engl J Med March 4, 2021; 384:e28. DOI: 10.1056/NEJMclde2101987. https://www.nejm.org/doi/full/10.1056/NEJMclde2101987
Nice case vignette on the question of whether we delay the second dose. Robert M. Wachter says yes, Nicole Lurie says no. No clear winner.
4 March
Paper of the Day
Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine — United States, February 2021. MMWR Morb Mortal Wkly Rep. ePub: 2 March 2021. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7009e4.htm
The Janssen COVID-19 vaccine is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector vaccine, encoding the stabilized prefusion spike glycoprotein of SARS-CoV-2. On February 27, 2021, the FDA issued an Emergency Use Authorization (EUA). This is an interim recommendation for use in persons aged ≥ 18 years for the prevention of COVID-19, discussing the available data and the question who to vaccinate and when. Answer: everybody, ASAP. Of note, the Janssen vaccine seems to work in the B.1.351 lineage from South Africa as well in the P.2 lineage from Brazil.
3 March
Paper of the Day
Saadat S, Tehrani ZR, Logue J, et al. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. March 1, 2021. doi:10.1001/jama.2021.3341. https://jamanetwork.com/journals/jama/fullarticle/2777171?resultClick=1
Health care workers with previous COVID-19, based on laboratory-confirmed serology testing, had higher antibody titer responses to a single dose of mRNA vaccine (Pfizer-BioNTech or Moderna, depending on personal preference and availability) than those who were not previously infected. Although the groups were small and only 59 volunteers were enrolled (17 in the Ab-negative, 16 in the asymptomatic, and 26 in the symptomatic group), this data indicates that one shot is enough in people with prior infection. Of note, titers did not differ between asymptomatic and symptomatic disease.
2 March
Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 Patients Requiring Mechanical Ventilation Following Implementation of a National COVID-19 Vaccination Program — Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep, 26 February 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7009e3external icon.
Again, encouraging data from Israel. The percentage of COVID-19 patients aged ≥ 70 years requiring mechanical ventilation fluctuated during October–December 2020 but has considerably and consistently decreased after implementation of the vaccination campaign prioritizing older adults. The decline in the ratio of persons aged ≥ 70 years to those < 50 years requiring mechanical ventilation began around the time of start of administration of the second dose of vaccine (January 10, 2021).
28 February
WHO 20210223. Weekly epidemiological update – 23 February 2021. WHO 2021, published 23 February. Full-text: https://www.who.int/publications/m/item/weekly-epidemiological-update—23-february-2021
Three pages about WHO’s current COVID-19 vaccine policy recommendations (page 4 to 6): age requirements, pregnant women, breastfeeding mothers, people with compromised immune system, living with HIV, or previously infected with SARS-CoV-2, people with a history of severe allergic reaction.
FDA 20210226. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Announcement. FDA 2021, published 26 February. Documents: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement#event-materials
Find here the documents that will lead to the approval of vaccine #4 – the one-shot adenovirus vector vaccine by Johnson & Johnson. The best news of the day: the vaccine had only a slightly reduced overall efficacy rate in South Africa (64% vs 72% in the US). Most importantly, the J&J vaccine showed 86% and 82% efficacy against severe disease of COVID-19 in the US and South Africa, respectively.
27 February
Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet February 25, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00502-X/fulltext
Early evidence for vaccine responses following previous natural infection. Maria Prendecki and colleagues looked at 72 HCWs from Imperial College Healthcare NHS Trust in London who were vaccinated, among them 21 (29%) participants with evidence of previous SARS-CoV-2 infection. Immune responses were analyzed 21-25 days after the first shot. In almost all individuals with previous SARS-CoV-2 infection, strong humoral and cellular responses to one dose of BNT162b2 vaccine, with evidence of high titers of virus neutralization were seen. In contrast, most infection-naive individuals generated only weak T cell responses and low titers of neutralizing antibodies.
Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet February 25, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00501-8/fulltext
Same direction: in this nested case-control analysis of 51 participants of COVIDsortium (24 seropositive) seronegative individuals had anti-S titers after one dose of vaccine comparable to peak anti-S titers in individuals with a previous natural infection who had not yet been vaccinated. Among those with a previous SARS-CoV-2 infection, vaccination increased anti-S titers more than 140-fold from peak pre-vaccine levels. This increase appears to be at least one order of magnitude greater than reported after a conventional prime-boost vaccine strategy in previously uninfected individuals.
Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. The Lancet Infectious Diseases, February 24, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00076-1/fulltext
Bell’s palsy is a type of facial paralysis that results in a temporary inability to control the facial muscles on the affected side of the face. Al Ozonoff and colleagues say that observed incidence of Bell’s palsy following mRNA vaccination is 3-7 times higher than would be expected in the general population. According to their comment, this signals a potential safety phenomenon and suggests inaccurate reporting to the public. However, it is also noted that Bell’s palsy usually self-resolves and that the mRNA vaccines offer a substantial net benefit to public health.
26 February
Paper of the Day
Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. NEJM February 24, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2101765
No doubt, paper of the day! Encouraging real-life data from Israel: Estimated vaccine effectiveness (> 1 M people vaccinated) during the follow-up period starting 7 days after the second dose was 92% for documented infection, 94% for symptomatic COVID-19, 87% for hospitalization, and 92% for severe COVID-19. Estimated effectiveness days 14 through 20 (after one dose) and days 21 through 27 (gradual shifting between the first and second vaccine doses) was 46% and 60% for documented infection, 57% and 66% for symptomatic COVID-19, 74% and 78% for hospitalization, 62% and 80% for severe COVID-19, and 72% and 84% for COVID-19–related death, respectively.
Kyriakidis NC, López-Cortés A, González EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 6, 28 (2021). https://www.nature.com/articles/s41541-021-00292-w
Comprehensive is right. A fantastic review discusses different strategies used for vaccine development and provides an overview of the current leading vaccine candidates against SARS-CoV-2.
22 February
Gee J, Marquez P, Su J, et al. First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. ePub: 19 February 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7008e3
From December 14, 2020 to January 13, 2021, a total of 13,794,904 COVID-19 vaccine doses were administered in the US. Anaphylaxis rates were comparable with those reported after receipt of other vaccines. No unexpected patterns of reactions or other safety concerns have been identified during early monitoring.
20 February
Amit S, Regev-Yochay G, Afek A, et al. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet February 18, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00448-7/fulltext
Good news from Israel, showing substantial early reductions in SARS-CoV-2 infection and symptomatic COVID-19 rates following first mRNA vaccine dose administration. Using a retrospective cohort of 9109 vaccine-eligible HCWs, Sharon Amit and colleagues estimate adjusted rate reductions of SARS-CoV-2 infections of 30% and 75% for days 1–14 and days 15–28 after the first dose, respectively.
Olliaro P. What does 95% COVID-19 vaccine efficacy really mean? Lancet Inf Dis February 17, 2021. Full-text: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00075-X/fulltext
Important note of caution: using simple mathematics, Piero Olliaro explains that 94–95% efficacy does not mean that 95% of people are protected from disease with the vaccine. This distinction is all the more important as we do not know whether and how it could vary if the vaccines were deployed on populations with different exposures, transmission levels, and attack rates.
19 February
Kupferschmidt K. Unprotected African health workers die as rich countries buy up COVID-19 vaccines. Science 2021, published 17 February. Full-text: https://www.sciencemag.org/news/2021/02/unprotected-african-health-workers-die-rich-countries-buy-covid-19-vaccines
While we are discussing how soon we can immunize everyone in the EU or the US, health workers continue to die in countries with zero doses administered so far.
18 February
Collier D, Ferreira I, Datir R, et al. Age-related heterogeneity in Neutralising antibody responses to SARS-CoV-2 following BNT162b2 vaccination. MedRxiv 2021, posted 16 February. Full-text: https://doi.org/10.1101/2021.02.03.21251054
Ravindra Gupta, Dami Collier and colleagues present a study of the immune response to the Pfizer/BioNTech vaccine in people aged 80 or older compared to younger people. Three weeks after the first dose a lower proportion of participants over 80 years old achieved an adequate neutralization titer of > 1:20 for 50% neutralization as compared to those under 80 (8/17 versus 19/24, p = 0,03); however, T cell responses were not different in those above or below 80 years. Following the second dose, 50% neutralizing antibody titers were above 1:20 in all individuals and there was no longer a difference by age.
Remmel A. COVID vaccines and safety: what the research says. Nature 2021, published 16 February. Full-text: https://www.nature.com/articles/d41586-021-00290-x
It is clear that coronavirus vaccines are safe and effective, but as more are rolled out, researchers are learning about the extent and nature of side effects.
17 February
Chua L, McPheeb R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021, published 9 February. Full-text: https://www.sciencedirect.com/science/article/pii/S0264410X21001535?via%3Dihub
Half-doses (50 μg) of Moderna’s mRNA-1273 vaccine might be as good as full doses (100 ug) at eliciting robust immune responses.
16 February
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021 Feb;21(2):e26-e35. PubMed: https://pubmed.gov/33125914. Full-text: https://doi.org/10.1016/S1473-3099(20)30773-8
The most important efficacy endpoint – protection against severe disease and death – is difficult to assess in Phase III clinical trials. In this review, Andrew Pollard, Susan Hodgson and colleagues explore the challenges in assessing the efficacy of candidate SARS-CoV-2 vaccines, discuss the caveats needed to interpret reported efficacy endpoints, and provide insight into answering the seemingly simple question, “Does this COVID-19 vaccine work?”. A must-read.
Rosenbaum L. Escaping Catch-22 — Overcoming Covid Vaccine Hesitancy. N Engl J Med 2021. Full-text: https://doi.org/10.1056/NEJMms2101220
Though many people initially believed a vaccine was the magic bullet that would save us from a devastating pandemic and return our lives to normalcy, we now find ourselves contemplating simultaneously how to ethically allocate a limited number of vaccine doses to the many people who want them and how to increase vaccine uptake among those who don’t.
21 Febuary
Paper of the Day
Voysey M, Costa Clemens SA, Madhi SA. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet February 19, 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00432-3/fulltext
In the case of the ChAdOx1 nCoV-19 (AZD1222) vaccine, it may be better to wait with the second shot. This pre-specified pooled analysis of AstraZeneca’s vaccine trials suggests that a 3-month dose interval might have advantages over a program with a shorter dosing interval. In the participants who received two standard doses, after the second dose, efficacy was higher in those with a longer prime-boost interval (vaccine efficacy 81% at ≥ 12 weeks) than in those with a short interval (vaccine efficacy 55% at < 6 weeks).
15 February
Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021 Feb 12. PubMed: https://pubmed.gov/33576785. Full-text: https://doi.org/10.1001/jama.2021.1967
During December 14, 2020 through January 18, 2021, a total of 9 943 247 doses of the Pfizer-BioNTech vaccine and 7 581 429 doses of the Moderna vaccine were reported administered in the US. CDC identified 66 case reports that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2 or 3): 47 following Pfizer-BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered.
McKie R. Life savers: the amazing story of the Oxford/AstraZeneca Covid vaccine. The Guardian 2021, published 14 February. Full-text: https://www.theguardian.com/world/2021/feb/14/life-savers-story-oxford-astrazeneca-coronavirus-vaccine-scientists
A year ago, Sarah Gilbert and Andrew Pollard began work on the response to a new virus. Now, as their vaccine is being given to millions, they tell of their incredible 12 months.
14 February
Krammer F, Srivastava K, the PARIS team, Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. MedRxiv 2021, posted 1 February. Full-text: https://doi.org/10.1101/2021.01.29.21250653
Should individuals who already had SARS-CoV-2 infection receive one or two shots of the currently authorized mRNA vaccines? Florian Krammer et al. remind us that the antibody response to the first vaccine dose in individuals with pre-existing immunity is equal to or even exceeds the titers found in naïve individuals after the second dose. They conclude that giving only one dose of vaccine would not negatively impact on antibody titers and free up many urgently needed vaccine doses.
Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature (2021). Full-text: https://doi.org/10.1038/s41586-021-03312-w
Victor Garcia, Angela Wahl and colleagues show that therapeutic and prophylactic administration of EIDD-2801 (Molnupiravir, MKK-4482), an oral broad spectrum antiviral currently in Phase II–III clinical trials, dramatically inhibits SARS-CoV-2 replication in vivo and thus has significant potential for the prevention and treatment of COVID-19.
13 February
(Another) Paper of the Day
Madhi A, Baillie VL, Cutland CL, et al. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa. medRxiv 2021, posted 12 February. Full-text: https://www.medrxiv.org/content/10.1101/2021.02.10.21251247v1
Shabir Madhi et al. report a ChAdOx1-nCoV19 (AstraZeneca) trial in HIV-uninfected people in South Africa. 23/717 (3.2%) placebo and 19/750 (2.5%) vaccine recipients developed mild-moderate Covid-19. Of the primary endpoint cases, 39/42 (92.9%) were the B.1.351 variant – against which vaccine efficacy was 10.4%. The authors conclude that a two-dose regimen of ChAdOx1-nCoV19 did not show protection against mild-moderate Covid-19 due to B.1.351 variant.
10 February
Petter E, Mor O, Zuckermann N, et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. GitHub 2021, posted 7 February. Full-text: http://bit.ly/3aPIetS
Vaccination with Pfizer-BioNTech’s Comirnaty could reduce the viral load by 1.6x to 20x in individuals who are positive for SARS-CoV-2. This estimate might improve after more individuals receive the second dose. Yaniv Erlich, Ella Petter and colleagues conclude that their findings indicate vaccination is not only important for an individual’s protection but can also reduce transmission.
Stamatatos L, Czartoski J, Wan YH, et al. Antibodies elicited by SARS-CoV-2 infection and boosted by vaccination neutralize an emerging variant and SARS-CoV-1. MedRxiv 2021, posted 8 February. Full-text: https://www.medrxiv.org/content/10.1101/2021.02.05.21251182v1
Andrew McGuire, Leonidas Stamatatos and colleagues found that a single shot of the Pfizer or Moderna mRNA vaccines boosts the neutralizing antibody response in people who were previously infected. Importantly, these antibodies also had neutralizing activity against the B1351 variant first detected in South Africa. The authors point to the importance of vaccination of both uninfected and of previously infected subjects. Read also Burton DR, Topo EJ. Toward superhuman SARS-CoV-2 immunity? Nat Med 27, 5–6 (2021). Full-text: https://doi.org/10.1038/s41591-020-01180-x
Burton DR, Topol EJ. Variant-proof vaccines — invest now for the next pandemic. Nature 2021, published 8 February. Full-text: https://www.nature.com/articles/d41586-021-00340-4
The rapid development and delivery of highly effective COVID-19 vaccines less than a year after the emergence of the disease is a huge success story. This was possible, in part, because of certain properties of the coronavirus SARS-CoV-2 that favor vaccine design — in particular, the spike protein on the viral surface. However, the next pathogen to emerge might be less accommodating. Eric Topol and Dennis Burton underline the importance of rational vaccine design based on broadly neutralizing antibodies.
Gadoth A, Halbrook M, Martin-Blais R, et al. Cross-sectional Assessment of COVID-19 Vaccine Acceptance Among Health Care Workers in Los Angeles. Ann Intern Med. 2021 Feb 9. PubMed: https://pubmed.gov/33556267. Full-text: https://doi.org/10.7326/M20-7580
In the context of a highly publicized coronavirus vaccine rollout, initial uptake by health care workers (HCWs) is critical for safety, health system functioning, and public opinion. In this survey, participants overwhelmingly acknowledged the importance and utility of general vaccination to a public health practice; however, they were widely hesitant about partaking in COVID-19 vaccination in either a trial or post-marketing settings and expressed uncertainties about the regulatory approval and protective capabilities of novel SARS-CoV-2 vaccines.
9 February
Paper of the Day
Levine-Tiefenbrun M, Yelin I, Katz R, et al. Decreased SARS-CoV-2 viral load following vaccination. MedRxiv 2021, posted 8 February. Full-text: https://www.medrxiv.org/content/10.1101/2021.02.06.21251283v1
Roy Kishony, Matan Levine-Tiefenbrun and colleagues analyzed positive SARS-CoV-2 test results following the first shot of the Pfizer-BioNTech vaccine. They found that the viral load is reduced 4-fold for infections occurring 12-28 days after the first dose of vaccine. These reduced viral loads might hint to lower infectiousness, further contributing to vaccine impact on virus spread.
Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 2021, published 8 February. Full-text: https://www.nature.com/articles/s41591-021-01270-4
In this in vitro study, the neutralization GMT of the serum panel against a virus with three mutations from the variant first detected in South Africa (E484K + N501Y + D614G) was slightly lower than the neutralization GMTs against a N501Y virus or a virus with three mutations from the UK variant (Δ69/70 + N501Y + D614G). The authors tested a panel of human sera from 20 participants in the Pfizer-BioNTech vaccine trial, drawn 2 or 4 weeks after immunization with two 30-μg doses of Comirnaty spaced 3 weeks apart.
8 February
News from Oxford
University of Oxford 20210207. ChAdOx1 nCov-19 provides minimal protection against mild-moderate COVID-19 infection from B.1.351 coronavirus variant in young South African adults. University of Oxford 2021, published 7 Febuary. Full-text: https://www.ox.ac.uk/news/2021-02-07-chadox1-ncov-19-provides-minimal-protection-against-mild-moderate-covid-19-infection
In an analysis, submitted as a pre-print prior to peer-review publication, a two-dose regimen of the ChAdOx1 nCoV-19 vaccine provides minimal protection against mild-to-moderate COVID-19 infection from the B1351 coronavirus variant first identified in South Africa.
7 February
Paper of the Day
Aran D. Estimating real-world COVID-19 vaccine effectiveness in Israel. GitHub 2021, posted 4 February. Full-text: https://github.com/dviraran/covid_analyses/blob/master/Aran_letter.pdf
This unpublished non-peer reviewed study suggests that the Pfizer-BioNTech vaccine might be between 66%-83% effective at preventing infection in individuals 60 years and older, 76-85% in those younger than 60 years, and 87-96% effective in preventing severe cases.
Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine Against SARS-CoV-2 VOC 202012/01 (B.1.1.7). Lancet Preprints 2021, posted 4 February. Full-text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3779160
This unpublished non-peer reviewed study by Andrew Pollard, Katherine Emary and colleagues reports that among participants in Phase II/III ChAdOx1 studies who had been infected with B117, vaccine efficacy against symptomatic SARS-CoV-2 infection was similar for B117 and non-B117 lineages (74,6% [95% CI: 41,6-88,9] and 84% [95% CI: 70,7-91,4], respectively). Importantly, virus neutralization activity by vaccine-induced antibodies was 9-fold lower against B117 than against a canonical non-B117 lineage.
6 February
Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. Patterns of covid-19 pandemic dynamics following deployment of a broad national immunization program. GitHub 2021, posted 3 February. Full-text: http://bit.ly/36KhjOU
SARS-CoV-2 vaccines work under real-world conditions. Eran Segal, Hagai Rossman and colleagues show that there was a 41% drop in COVID-19 infections in people aged 60 or older from mid-January to early February. During the same period, there was also a 31% drop in hospitalizations (Rossmann 2021, Figure 11). In people aged 59 and younger who received the vaccine later, cases dropped by only 12% and hospitalizations by 5%.
5 February
Voysey M, Costa Clemens SA, Madhi SA, et al. Single Dose Administration, And The Influence Of The Timing Of The Booster Dose On Immunogenicity and Efficacy Of ChAdOx1 nCoV-19 (AZD1222) Vaccine. Lancet Preprints 2021, posted 1 February. Full-text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3777268
Andrew Pollard, Sarah Gilbert, Merryn Voysey and colleagues present data from Phase III efficacy trials of ChAdOx1 nCoV-19 in the United Kingdom and Brazil, and Phase I/II clinical trials in the UK and South Africa. They report that vaccine efficacy after a single standard dose of vaccine from day 22 to day 90 post vaccination was 76% (59%, 86%), and that protection did not wane during this initial 3-month period. The authors conclude that vaccination programs aimed at vaccinating a large proportion of the population with a single dose, with a second dose given after a 3 month period is an effective strategy for reducing disease, and may be optimal for rollout of a pandemic vaccine when supplies are limited.
Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021, published 3 February. Full-text: https://doi.org/10.1016/S1473-3099(20)30987-7
Weidong Yin, Yuliang Zhao, Zhiwei Wu and colleagues report the results of Sinovac’s CoronaVac safety and immunogenicity data in adults aged 60 years or older (previous publications: Zhang Y 2020,
Gao 2020). Reminder: CoronaVac™ is an inactivated virus vaccine. On 12 January, the government of São Paulo, Brazil, announced the overall effectiveness of the Sinovac vaccine to be 50,38%. The data was obtained with tests carried out on 12.508 volunteers in the country, all health professionals. According to a report of The New York Times (7 January), Sinovac has sold more than 300 million doses, mostly to low- and middle-income countries, accounting for about half of the total production of Sinovac. See also COVID Reference Vaccines.
Gerberding JL, Haynes BF. Vaccine Innovations — Past and Future. N Engl J Med 2021; 384:393-396. Full-text: Full-text: https://doi.org/10.1056/NEJMp2029466
A 4-page overview by Julie Gerberding and Barton Haynes. The authors anticipate that the future holds great promise for vaccine-mediated control of global pathogens but providing affordable access to effective vaccines for everyone who could benefit from them remains an important challenge. Difficulties facing vaccinologists now include
- predicting the type and timing of the next pandemic;
- developing vaccines to combat rapidly changing pathogens such as HIV-1, influenza, and multidrug-resistant bacteria;
- and establishing rapid-response strategies to control emerging and reemerging infectious diseases.
4 February
Saadat S, Rikhtegaran-Tehrani Z, Logue J, et al. Single Dose Vaccination in Healthcare Workers Previously Infected with SARS-CoV-2. medRxiv 2021, posted 1 February. Full-text: https://doi.org/10.1101/2021.01.30.21250843
The same direction: Healthcare workers (HCW) with prior COVID-19 showed clear secondary antibody responses to vaccination with IgG spike binding titers rapidly increasing by 7 days and peaking by days 10 and 14 post-vaccination. The authors’ conclusion: in times of vaccine shortage 1) a single dose of vaccine for patients already having had laboratory-confirmed COVID-19; and 2) patients who have had laboratory-confirmed COVID-19 can be placed lower on the vaccination priority list.
3 February
Paper of the Day
Logunov DY, Dolzhikova IV, Shcheblyakov DV et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, published 2 February. Full-text: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00234-8/fulltext
A new entry in the COVID-19 Vaccine Club (CVC): Denis Logunov and colleagues report on the interim clinical efficacy results of the Russian Sputnik V vaccine (rAd26 and rAd5). From 21 days after the first dose of vaccine, 16 (0·1%) of 14 964 participants in the vaccine group and 62 (1·3%) of 4902 in the placebo group were confirmed to have COVID-19; vaccine efficacy was 91·6% (95% CI 85·6–95·2). The vaccine will help to diversify the world SARS-CoV-2 vaccine pipeline. Welcome to the CVC, Sputnik V!
See also Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, published 2 February. Full-text: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00191-4/fulltext
Krammer F, Srivastava K, PARIS team, Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv 2021, posted 1 February. Full-text: https://doi.org/10.1101/2021.01.29.21250653
Individuals with pre-existing immunity against SARS-CoV-2 need only one dose of vaccine. Consequence: we could spare the second for other people. This is the result of a study by Florian Krammer and colleagues who provide evidence that the antibody response to the first vaccine dose in individuals with pre-existing immunity is equal to or even exceeds the titers found in naive individuals after the second dose. They also show that the reactogenicity is significantly higher in individuals who were previously infected with SARS-CoV-2. See also Willyard C. Had Covid? You May Need Only One Dose of Vaccine, Study Suggests. The New York Times 2021, published 1 February. Full-text: https://www.nytimes.com/2021/02/01/health/have-you-had-covid-19-coronavirus.html
Vogel AB, Kanevsky I, Che Y, et al. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021 Feb 1. PubMed: https://pubmed.gov/33524990. Full-text: https://doi.org/10.1038/s41586-021-03275-y
The people from BioNTech talk about the beginnings of the vaccine developed now in cooperation with Pfizer. This Nature paper reports the first antigen-specific immune responses in… mice and rhesus macaques.
31 January
Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science 2021, published 29 January. Full-text: https://doi.org/10.1126/science.abg6105
The authors tested SARS-CoV-2-S pseudoviruses bearing either the Wuhan reference strain or the B.1.1.7 lineage spike protein with sera of 40 participants who were vaccinated in a previously reported trial with the Pfizer-BioNTech mRNA-based vaccine Comirnaty. The immune sera had slightly reduced but overall largely preserved neutralizing titers against the B.1.1.7 lineage pseudovirus. These data indicate that the B.1.1.7 lineage will not escape BNT162b2-mediated protection.
Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, published 29 January. Full-text: https://doi.org/10.1016/S0140-6736(21)00241-5
Ralf Clemens, Peter Richmond and colleagues show that the Clover Biopharmaceuticals SCB-2019 vaccine (comprising S-Trimer protein formulated with either AS03 [GSK] or CpG/Alum adjuvants), elicited robust humoral and cellular immune responses against SARS-CoV-2, with high viral neutralizing activity. See also comment by Blakney AK, McKay PF. Next-generation COVID-19 vaccines: here come the proteins. Lancet 2021, published 29 January. Full-text: https://doi.org/10.1016/S0140-6736(21)00258-0
Callaway E, Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature 2021, published 29 January. Full-text: https://www.nature.com/articles/d41586-021-00268-9
Novavax’s experimental shot is highly effective against the variant identified in Britain — but saw a worrying drop in efficacy against a lineage detected in South Africa.
29 January
Wang P, Lihong L, Iketani S, et al. Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization. bioRxiv 2021, posted 26 January. Full-text: https://doi.org/10.1101/2021.01.25.428137
E484K is the bad boy on the block. David Ho, Pengfai Wang and colleagues at Columbia University produced retroviruses with spike proteins incorporating each of B1351’s mutations separately, as well as all at once. E484K accounted for much of the effect. The serum of 12 people vaccinated with Moderna’s vaccine and 10 people vaccinated with the Pfizer-BioNTech vaccine was six to nine times less potent against B.1.351. Serum from 20 previously infected people was 11 to 33 times less potent.
Kupferschmidt 20210126. Vaccine 2.0: Moderna and other companies plan tweaks that would protect against new coronavirus mutations. Science 2021, published 26 January. Full-text: https://www.sciencemag.org/news/2021/01/vaccine-20-moderna-and-other-companies-plan-tweaks-would-protect-against-new
Antibodies triggered by the vaccine may be a little less potent against the new variant B.1.351, first described in South Africa, vs the one the vaccine was developed for. So, researchers were perhaps relieved to hear the company will start development of booster shots tailored to B.1.351 and other variants.
Brunning A. How are RNA vaccines made? Periodic Graphics 2021, published 3 January. Link: https://cen.acs.org/pharmaceuticals/vaccines/Periodic-Graphics-RNA-vaccines-made/99/i1
RNA vaccines produced by Pfizer-BioNTech and Moderna have become the first COVID-19 vaccines. How are these vaccines made?
28 January
Brouwer JM, Brinkkemper M, Maisonnasse P, et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 2021, published 25 January. Full-text: https://www.cell.com/cell/fulltext/S0092-8674(21)00078-7
Rogier Sanders, Philip Brouwer and colleagues present a two-component protein-based nanoparticle vaccine that displays twenty copies of pre-fusion SARS-CoV-2 S protein, capable of inducing potent neutralizing antibody responses in 400 in mice, rabbits and cynomolgus macaques. The vaccine-induced immunity protected macaques against a high dose challenge, resulting in strongly reduced viral infection and replication in upper and lower airways.
Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. bioRxiv 2021, posted 19 January. Full-text: https://doi.org/10.1101/2021.01.18.426984
Good news from the variants vaccine front. Ugur Sahin, Alexander Muik and colleagues report that after analyzing immune sera from individuals vaccinated with the Pfizer-BioNTech vaccine (Comirnaty™), it seems unlikely that the (“UK”) B.1.1.7 variant will escape vaccine-mediated protection. The authors investigated SARS-CoV-2-S pseudoviruses bearing either the Wuhan reference strain or the B.1.1.7 lineage spike protein with the sera of 16 participants in a previously reported trial with the mRNA-based COVID-19 vaccine Comirnaty™. The immune sera had equivalent neutralizing titers to both variants.
Wu K, Werner AP, Moliva JI, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv 2021, published 25 January. Full-text: https://doi.org/10.1101/2021.01.25.427948
Good news from another variants vaccine front. Kai Wu et al. demonstrate that people aged 18-55 years who received two 100 µg doses of the mRNA-1273 vaccine, “maintained activity against all circulating strain variants tested to date”, and only the B.1.351 variant showed reduced neutralizing titers. Viral escape was not detected from any sample and neutralizing titers remained above those previously found to be protective in NHP challenge studies. (Editor’s Note: All circulating strain variants? The paper doesn’t seem to mention the P.1 variant from Brazil.) See also the Moderna press release at https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against
Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 variants from neutralization by convalescent plasma. medRxiv 2021, published 26 January. Full-text: https://doi.org/10.1101/2021.01.26.21250224
Bad news from the variants antibody front. Mutations in the B.1.351 variant (alias 501Y.V2) may cause the virus to lose much of its sensitivity to antibodies. That is the result of a pre-print paper by Tulio de Oliveira, Alex Sigal, Sandile Cele and colleagues. After examining the neutralizing effect of convalescent plasma collected from six adults hospitalized with COVID-19, the authors observed that neutralization of the B.1.351 variant was strongly attenuated, with IC50 6 to 200-fold higher relative to the first wave virus. Reduced protection against re-infection? Let’s live on and see!
Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv 2021, published 19 January. Full-text: https://doi.org/10.1101/2021.01.15.426911
And, finally, good news again: Michel Nussenzweig and colleagues tested samples from 14 and 6 people who had received the Moderna and the Pfizer-BioNTech vaccine, respectively. They saw a slight decrease in antibody activity against engineered viruses with three key mutations of the B.1.351 variant first discovered in South Africa.
26 January
CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine — United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. ePub: 22 January 2021. Full-text: http://dx.doi.org/10.15585/mmwr.mm7004e1external icon.
Two in a Million: from December 21, 2020 to January 10, 2021, CDC detected 10 cases of anaphylaxis after administration of a reported 4.041.396 first doses of the Moderna COVID-19 vaccine (2.5 cases per million doses administered). No anaphylaxis-related deaths were reported. Nine events occurred in persons with a documented history of allergies or allergic reactions, five of whom had a previous history of anaphylaxis. The median interval from vaccine receipt to symptom onset was 7.5 minutes. Nine patients had onset within 15 minutes, and one had onset after 30 minutes.
24 January
Bubar KM, Reinholt K, Kissler SM, et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 21 Jan 2021:eabe6959. DOI: 10.1126/science.abe6959
Mathematical models comparing five age-stratified prioritization strategies: a highly effective transmission-blocking vaccine prioritized to adults ages 20-49 years minimized cumulative incidence, but mortality and years of life lost were minimized in most scenarios when the vaccine was prioritized to adults over 60 years old. Use of individual-level serological tests to redirect doses to seronegative individuals improved the marginal impact of each dose while potentially reducing existing inequities in COVID-19 impact.
Fitzpatrick MC, Galvani AP. Optimizing age-specific vaccination. Science 21 Jan 2021: eabg2334. DOI: 10.1126/science.abg2334
Vaccination strategies are not one size fits all. In their perspective, Meagan C. Fitzpatrick and Alison P. Galvani looked at vaccination of different age groups. Although vaccination of younger adults is projected to avert the greatest incidence, vaccinating older adults will most effectively reduce mortality.
23 January
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature News 21 January 2021. Full-text: https://www.nature.com/articles/d41586-021-00121-z
Ewen Callaway discusses the growing evidence that the SARS-CoV-2 variant identified in South Africa might compromise immunity sparks concerns about vaccine effectiveness.
Ella E, Vadreva KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis January 21, 2021. Full-text: https://doi.org/10.1016/S1473-3099(20)30942-7
In this double-blind, multi-center, randomized Phase I trial from India, the inactivated vaccine BBV152 led to tolerable safety outcomes and enhanced immune responses. Different adjuvants were also evaluated (chemosorbed imidazoquinoline onto the aluminum hydroxide gel or not). In 375 participants who were assigned to receive two doses separated by 2 weeks of BBV152 3 μg with Algel-IMDG (n = 100), 6 μg with Algel-IMDG (n = 100), or 6 μg with Algel (n = 100), or an Algel-only control (n = 75), 80% of patients in each vaccine group seroconverted, with at least a four-fold increase in binding antibody titers. Seroconversion occurred by microneutralization in 88% and 92% of the 3 and 6 μg Algel-IMDG groups but also in 8% of the control group, suggesting SARS-CoV-2 infections occurred in some participants.
Rostad CA, Anderson EJ. Optimism and caution for an inactivated COVID-19 vaccine. Lancet Inf Dis January 21, 2021. Full-text: https://doi.org/10.1016/S1473-3099(20)30988-9
Christina Rostad and Evan Anderson see an inactivated vaccine as a welcome addition to the COVID-19 vaccine landscape. However, they discuss the open questions and concerns regarding inactivated vaccines (i.e. antibody-dependent enhancement of infection and vaccine-associated enhanced respiratory disease). Until then, they “will wait with cautious optimism on this vaccine candidate poised to bolster worldwide equitable access to COVID-19 prevention”. Ok, let’s wait. But for how long?
Siegel CA, Melmed GY, McGovern DP, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021 Jan 20:gutjnl-2020-324000. PubMed: https://pubmed.gov/33472895. Full-text: https://doi.org/10.1136/gutjnl-2020-324000
The panel recommends vaccinating all patients with IBD as soon as they are able to receive the vaccine, regardless of immune-modifying therapies. The exception is for any live-attenuated virus vaccines or replication-competent viral vector vaccines that come to market.
22 January
Klass P, Ratner AJ. Vaccinating Children against Covid-19 — The Lessons of Measles. NEJM January 20, 2021. Full-text: https://doi.org/10.1056/NEJMp2034765
Perri Klass and Adam Ratner argue that we need to consider lessons from recent measles epidemics — not only about the power of legislative mandates, but also about their potential for sowing distrust if delivered without careful, sensitive, accurate public health messaging. Communication, people!
Connors M, Graham BS, Lane HC, Fauci AS. SARS-CoV-2 Vaccines: Much Accomplished, Much to Learn. Ann Intern Med. 2021 Jan 19. PubMed: https://pubmed.gov/33460347. Full-text: https://doi.org/10.7326/M21-0111
Progress toward effective vaccines for SARS-CoV-2 has proceeded at an unprecedented pace and it is highly likely that vaccination and its subsequent ability to prevent disease will provide critical and life-saving benefit in the coming months and may be one of our surest ways to emerge from this pandemic to a more normal society. However, acknowledging that there is still much to learn while strongly encouraging vaccination is a critical challenge facing health care today.
20 January
Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med (2021). Full-text: https://doi.org/10.1038/s41591-021-01230-y
After Phase III vaccine trials, a comprehensive program of prevention, continued work on vaccine optimization, new vaccines, correlates, long-term safety and continued surveillance will be needed simultaneously with the steady implementation of vaccination.
Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid-19 in people who report allergies. BMJ. 2021 Jan 18;372:n120. PubMed: https://pubmed.gov/33461962. Full-text: https://doi.org/10.1136/bmj.n120
A history of severe allergy does not preclude vaccination unless that allergy is to the vaccine or its components. Discover the key facts and reassure your patients.
18 January
Topol EJ. Messenger RNA vaccines against SARS-CoV-2. Cell 2021, published 16 January. Access: https://www.cell.com/cell/fulltext/S0092-8674(20)31761-X
The first two vaccines proven to be effective for inhibiting COVID-19 illness were both mRNA, achieving 95% efficacy (and safety) among 74.000 participants (half receiving placebo) after intramuscular delivery of two shots, 3–4 weeks apart. A one-page summary by Eric Topol.
16 January
Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun 12, 372 (2021). Full-text: https://doi.org/10.1038/s41467-020-20653-8
In mice and baboons, low-dose levels of NVX-CoV2373 with Matrix-M was highly immunogenic. NVX-CoV2373, developed by Novavax, is a recombinant nanoparticle vaccine (rSARS-CoV-2) composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant. Find more information about NVX-CoV2373 at https://covidreference.com/vaccines.
Ledford H. How can countries stretch COVID vaccine supplies? Scientists are divided over dosing strategies. Nature 2021, published 11 January. Full-text: https://www.nature.com/articles/d41586-021-00001-6
On 30 December, the United Kingdom announced that it would allow doses of two coronavirus vaccines to be administered as many as 12 weeks apart, even though, in clinical trials, the two doses of the vaccine made by Pfizer of New York City and BioNTech of Mainz, Germany, were given to participants about three weeks apart. Not everyone agrees.
15 January
Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med 2021, published 13 January. Full-text: https://doi.org/10.1056/NEJMoa2034201
Hanneke Schuitemaker, Jerald Sadoff and colleagues describe the safety and immunogenicity profiles of Ad26.COV2.S. As with other SARS-CoV-2 vaccines, the most frequent adverse events were fatigue, headache, myalgia, and injection site pain. Systemic adverse events were less common in people 65 years or older than in those 18 to 55 years of age. Neutralizing-antibody titers against wild type virus were detected in 90% or more of all participants on day 29 after the first vaccine dose and reached 100% by day 57 with a further increase in titers (GMT, 288 to 488), regardless of vaccine dose or age group. Ad26.COV2.S, developed by Janssen Pharmaceutical Companies of Johnson & Johnson, is a recombinant replication-incompetent adenovirus type 26 (Ad26) vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen.
Efrati I. Israel to Share Vaccination Data With Pfizer as Part of Secret Deal. Haaretz 2021, published 10 January. Full-text: https://www.haaretz.com/israel-news/.premium-israel-to-share-covid-vaccine-data-with-pfizer-but-agreement-remains-secret-1.9438504
The Israeli newspaper reports a deal between Israel and Pfizer: Pfizer will receive anonymized data about consequences of the inoculations, side effects, efficacy, and the amount of time it takes to develop antibodies, according to different types of population, age, gender, pre-existing conditions, and other factors.
14 January
Dolgin E. How COVID unlocked the power of RNA vaccines. Nature 2021, published 12 January. Full-text: https://www.nature.com/articles/d41586-021-00019-w
The technology could revolutionize efforts to immunize against HIV, malaria, influenza and more.
Mahase E. How the Oxford-AstraZeneca covid-19 vaccine was made. BMJ 2021; 372. Full-text: https://doi.org/10.1136/bmj.n86
Andrew Pollard has been leading the Oxford vaccine clinical trials in the UK, Brazil, and South Africa. He tells how the Oxford vaccine came to be, how dosing was worked out, and whether it will stand up to the new variants.
13 January
Marjot T, Webb GJ, Barritt AS. SARS-CoV-2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol 2021, published 11 January. Full-text: https://doi.org/10.1016/S2468-1253(21)00008-X
Patients with advanced liver disease have well recognized deficiencies in innate and humoral immunity, termed cirrhosis-associated immune dysfunction (CAID). Nonetheless, given the high COVID-19-related mortality in patients with decompensated cirrhosis, it remains of utmost importance to prioritize vaccinations in this sub-group. Eleanor Barne, Thomas Marjot and colleagues express their belief that patients with advanced liver disease should be prioritized for vaccination, with the likely benefits far outweighing any potential risks. Until it is established whether patients with liver disease and transplantation achieve optimal protection after immunization, clinicians should remain vigilant for post-vaccination COVID-19 in these cohorts.
12 January
Bordon, Y. Immune readouts from the Oxford COVID-19 vaccine. Nat Rev Immunol 2021, published 11 January. Full-text: https://doi.org/10.1038/s41577-021-00503-4
Yvonne Bordon comments on two recent reports from the Oxford COVID-19 vaccine team which detail the immune outcomes observed in a Phase I/II trial of their ChAdOx1 nCoV-19 vaccine. In one of the papers we presented on 21 December, the authors give a detailed description of the immune response after administration of one dose of ChAdOx1 nCoV-19 in 88 adults (ages 18-55 years) (Ewer 2020). They define the isotypes, subclasses and antibody avidity induced after vaccination. They also performed multiplex cytokine profiling and intracellular cytokine staining analysis, demonstrating that ChAdOx1 nCoV-19 vaccination induces a predominantly Th1-type response (that appears to be good). See also Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2020 Dec 17. PubMed: https://pubmed.gov/33335322. Full-text: https://doi.org/10.1038/s41591-020-01179-4
11 January
Xie X, Zou J, Fonte-Garfias CR, et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv 2021, posted 7 January. Full-text: https://doi.org/10.1101/2021.01.07.425740
Recent SARS-CoV-2 variants in the United Kingdom and South Africa have multiple mutations in their S glycoproteins, which are key targets of viral neutralizing antibodies. These rapidly spreading variants share the spike N501Y substitution. Pei-Yong Shi, Philip R. Dormitzer and Xuping Xie generated isogenic N501 and Y501 SARS-CoV-2. Sera of participants in a previously reported trial of the mRNA-based COVID-19 vaccine BNT162b2 had equivalent neutralizing titers to the N501 and Y501 viruses.
10 January
Wood S, Schulman K. Beyond Politics — Promoting Covid-19 Vaccination in the United States. N Engl J Med 2021, published 6 January. Full-text: https://doi.org/10.1056/NEJMms2033790
In France, some 60% of the population is skeptic about getting vaccinated. In search of inspiration, French authorities should take a look at this paper by Stacy Wood & Kevin Schulman. They should be ready to revise their marketing skills. Communication needs to become more a part of regular science.
EMA 20201221. Comirnaty. European Medicines Agency 2020, published 23 December. Full-text: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty
Find the 32-page product information of the EMA.
EMA 20210106. COVID-19 Vaccine Moderna. European Medicines Agency 2021, published 6 January. Full-text: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/covid-19-vaccine-moderna
Find the product information as approved by the CHMP on 6 January 2021, pending endorsement by the European Commission.
Garde D. ‘I haven’t even told my wife’. Stat 2020. Full-text: https://www.statnews.com/2020/12/15/inside-the-frantic-and-secretive-sprint-to-name-the-covid-19-vaccines/
Naming a vaccine is almost always a matter of threading semantic needles, branding experts said, where the goal is to evoke positive vibes without irking the world’s more conservative regulatory bodies. And it takes time.
9 January
Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature 2021, published 8 January. Full-text: https://www.nature.com/articles/d41586-021-00031-0
Researchers race to determine why variants identified in Britain and South Africa spread so quickly and whether they’ll compromise vaccines.
Graph
8 January
CDC 20210106. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. ePub: 6 January 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7002e1
One case of anaphylaxis in 100.000 vaccine recipients of the BioNTech/Pfizer vaccine Comirnaty. That is the result of the 10 days of monitoring (14-23 December) by the Vaccine Adverse Event Reporting System which detected 21 cases of anaphylaxis after administration of a reported 1.893.360 first doses of the vaccine (11,1 cases per million doses). Note that 71% of these occurred within 15 minutes of vaccination. Screen recipients for contraindications and precautions; have the necessary supplies available to manage anaphylaxis; implement the recommended post-vaccination observation periods; and immediately treat suspected cases of anaphylaxis with intramuscular injection of epinephrine! For detailed insight, check also Castells MC, Phillips EJ. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med 2020, published 30 December. Full-text: https://doi.org/10.1056/NEJMra2035343 which we presented on 1 January.
Iacobucci G, Mahase E. Covid-19 vaccination: What’s the evidence for extending the dosing interval? BMJ 2021, published 6 January. Full-text: https://doi.org/10.1136/bmj.n18
On 30 December the four UK chief medical officers announced that the second doses of the COVID vaccines should be given towards the end of 12 weeks rather than in the previously recommended 3-4 weeks. German authorities will issue a similar recommendation soon. Gareth Iacobucci and Elisabeth Mahase look at the questions this has raised.
5 January
De Vrieze J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science 2020, published 21 December (sorry for being late!). Full-text: https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions
Severe allergy-like reactions in at least eight people who received the COVID-19 vaccine produced by Pfizer and BioNTech over the past 2 weeks may be due to a compound in the packaging of the messenger RNA (mRNA) that forms the vaccine’s main ingredient, scientists say. A similar mRNA vaccine developed by Moderna, which was authorized for emergency use in the United States on Friday, also contains the compound, polyethylene glycol (PEG).
4 January
CDC 20201231. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. Vaccines & Immunizations 2020, last reviewed: December 31, 2020. Full-text: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/anaphylaxis-management.html
Anaphylaxis has been reported following COVID-19 vaccination. The incidence of anaphylaxis associated with the Pfizer SARS-CoV-2 mRNA vaccine appears to be approximately 10 times as high as the incidence reported with all previous vaccines, at approximately 1 in 100,000, as compared 1 in 1,000,000 (Castells 2020). The CDC recommends that appropriate medical treatment for severe allergic reactions must be immediately available in the event that an acute anaphylactic reaction occurs following administration of an mRNA COVID-19 vaccine. In particular, persons without contraindications to vaccination who receive an mRNA COVID-19 vaccine be observed after vaccination for the following time periods:
- 30 minutes: Persons with a history of an immediate allergic reaction of any severity to a vaccine or injectable therapy and persons with a history of anaphylaxis due to any cause.
- 15 minutes: All other persons
3 January
Editorial. Messengers of hope. Nat Biotechnol. 2020 Dec 29:1. PubMed: https://pubmed.gov/33376248. Full-text: https://doi.org/10.1038/s41587-020-00807-1
Emergency Use Authorizations for two mRNA COVID-19 vaccines represent a turning point in the pandemic. They also herald a new era for vaccinology – an era where vaccines are designed on computers, iteratively optimized in discovery and manufactured on demand — all without expensive and finicky cell culture.
Oliver S, Gargano J, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1653-1656. Full-text: http://dx.doi.org/10.15585/mmwr.mm695152e1
Use of all COVID-19 vaccines authorized under an EUA should be implemented in conjunction with ACIP’s interim recommendations for allocating initial supplies of COVID-19 vaccines. Before vaccination, the EUA Fact Sheet should be provided to recipients and caregivers. Providers should counsel Moderna COVID-19 vaccine recipients about expected local and systemic reactogenicity.
1 January
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2020, published 30 December. Full-text: https://doi.org/10.1056/NEJMoa2035389
Finally – after press releases, an emergency use authorization and the start of mass vaccinations – the scientific paper by Lindsey Baden et al.! Nothing new: the mRNA-1273 vaccine developed by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), within the National Institutes of Health (NIH), has more than 90% efficacy at preventing COVID-19 illness, including severe disease. Moderate-to-severe systemic side effects, such as fatigue, myalgia, arthralgia, and headache, were noted in about 50% of participants in the mRNA-1273 group after the second dose. These side effects were transient, starting about 15 hours after vaccination and resolving in most participants by day 2, without sequelae. The incidence of serious adverse events reported throughout the entire trial was similar for mRNA-1273 and placebo. Importantly, mRNA-1273 did not show evidence in the short term of enhanced respiratory disease after infection, a concern that had emerged from animal models used in evaluating SARS and Middle East Respiratory Syndrome (MERS) vaccine constructs. The authors rightly conclude that the safety of the mRNA-1273 vaccine regimen seems to be reassuring.
Castells MC, Phillips EJ. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med 2020, published 30 December. Full-text: https://doi.org/10.1056/NEJMra2035343
On December 8, 2020, within 24 hours after the start of the U.K. mass vaccination program for health care workers and elderly adults, the program reported probable cases of anaphylaxis in two women, 40 and 49 years of age, who had known food and drug allergies and were carrying auto-injectable epinephrine. One week later, a 32-year-old female health care worker in Alaska who had no known allergies presented with an anaphylactic reaction within 10 minutes after receiving the first dose of the vaccine. Since then, several more cases of anaphylaxis associated with the Pfizer mRNA vaccine have been reported in the United States after vaccination of almost 2 million health care workers, and the incidence of anaphylaxis associated with the Pfizer SARS-CoV-2 mRNA vaccine appears to be approximately 10 times as high as the incidence reported with all previous vaccines, at approximately 1 in 100,000, as compared 1 in 1,000,000. Mariana Castells and Elizabeth Phillips explain what is at stake: “It is critical that we focus on safe and efficient approaches to implementing mass vaccination. In the future, these new vaccines may mark the beginning of an era of personalized vaccinology in which we can tailor the safest and most effective vaccine on an individual and a population level.” Happy New Year!
31 December
GOV.UK 20201230. Regulatory approval of COVID-19 Vaccine AstraZeneca. https://www.gov.uk 2020, published 30 December. Full-texts: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca
On December 30, UK regulatory authorities approved the Oxford University/AstraZeneca vaccine. ChAdOx1 nCoV-19 (AZD1222) needs only normal refrigeration at 2-8°C and is far cheaper than the previously approved vaccines Comirnaty (BioNTech/Pfizer) and mRNA-1273 (Moderna).
Joint Committee on Vaccination and Immunisation. JCVI issues advice on the AstraZeneca COVID-19 vaccine. JCVI 2020, published 30 December 2020. Full-text: https://www.gov.uk/government/news/jcvi-issues-advice-on-the-astrazeneca-covid-19-vaccine
The Joint Committee on Vaccination and Immunisation (JCVI) recommends that both the AstraZeneca (ChAdOx1) and the BioNTech/Pfizer (Comirnaty) vaccines are safe and provide high-levels of protection against COVID-19, including severe COVID-19. As protection is obtained around 2 weeks after the first vaccine dose, the committee recommends that vaccinating more people with the first dose is prioritized above offering others their second dose. This would provide the greatest public health benefits in the short term and save more lives. For the BioNTech/Pfizer vaccine, the second vaccine dose can be offered between 3 to 12 weeks after the first dose. (Pfizer is not amused.) For the AstraZeneca vaccine, the second dose can be offered 4 to 12 weeks after the first dose.
Our World in Data. COVID-19 vaccination doses administered per 100 people. 30 December 2020. Link: https://ourworldindata.org/grapher/covid-vaccination-doses-per-capita
28 December
The Pfizer–BioNTech COVID-19 vaccine, codenamed BNT162b2, is now being sold under the brand name Comirnaty™ (INN: Tozinameran). Find here a summary of the product characteristics and the package leaflet if you want to be able to answer the questions of your patients: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf
27 December
Burki T. Equitable distribution of COVID-19 vaccines. Lancet Infect Dis 2021, published 1 January. Full-text: https://doi.org/10.1016/S1473-3099(20)30949-X
If everything goes according to plan, November 2020 will be remembered as the beginning of the end of the COVID-19 pandemic. Countries will have to ensure that they have the infrastructure for mass immunization campaigns. Those without experience in distributing influenza vaccines must learn how to establish platforms for adult vaccination. Vaccine hesitancy will have to be overcome.
Lucia VC, Kelekar A, Afonso NM. COVID-19 vaccine hesitancy among medical students, Journal of Public Health. J Pub Health 2020, published 26 December. Full-text: https://doi.org/10.1093/pubmed/fdaa230
Medical students are among the group of frontline healthcare providers likely to be exposed to COVID-19 patients. It is important to achieve high COVID-19 vaccination coverage rates in this group as soon as a vaccine is available. In this survey completed by 168 of 494 medical students (response rate = 34%), the vast majority had positive attitudes regarding immunizations in general and the importance of vaccines for themselves and patients. 53% indicated they would participate in a COVID-19 vaccine trial; only 23% were unwilling to take a COVID-19 vaccine immediately upon FDA approval.
24 December
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol (2020). Full-text: https://doi.org/10.1038/s41577-020-00479-7
2021 may be the perfect time for immunologists to be involved in designing the next generation of powerful immunogens. In this review, Andrew Pollard and Else Bijker provide an overview of vaccines, immunization and related issues.
23 December
Oliver S, Gargano J, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. ePub: 20 December 2020. DOI: http://dx.doi.org/10.15585/mmwr.mm695152e1
The Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Moderna COVID-19 (mRNA-1273) vaccine. Adverse events that occur in a recipient after receipt of COVID-19 vaccine should be reported to the Vaccine Adverse Events Reporting System (VAERS). FDA requires that vaccination providers report vaccination administration errors, serious adverse events, cases of multisystem inflammatory syndrome, and cases of COVID-19 that result in hospitalization or death after administration of the COVID-19 vaccine under an EUA. Information on how to submit a report to VAERS is available at https://vaers.hhs.gov/index.html.
22 December
Ball P. The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature 2020, published 18 December. Full-text: https://www.nature.com/articles/d41586-020-03626-1
The speedy approach used to tackle SARS-CoV-2 could change the future of vaccine science. “It shows how fast vaccine development can proceed when there is a true global emergency and sufficient resources,” says Dan Barouch, director of the Center for Virology and Vaccine Research at Harvard Medical School in Boston, Massachusetts.
Rubin EJ, Baden LR, Barocas JA, Morrissey S. Covid-19 Vaccine Fundamentals. Audio interview (19:46). N Engl J Med 2020; 383: e146. Access: https://doi.org/10.1056/NEJMe2035370
The editors discuss the five types of COVID-19 vaccine under study, as well as trial results of the therapeutic agent baricitinib.
21 December
Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol 18 December 2020. Full-text: https://doi.org/10.1038/s41577-020-00480-0
Which viral elements are used in COVID-19 vaccine candidates, why might they act as good targets for the immune system and what are the implications for protective immunity? This review addresses these questions.
Ewer KJ, Barrett JR, Belij-Rammerstorfer S. et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med December 18, 2020. Full-text: https://doi.org/10.1038/s41591-020-01194-5
A detailed description of the immune response after administration of one dose of ChAdOx1 nCoV-19 in 88 adults (ages 18-55 years). The authors define, in detail, the isotypes, subclasses and antibody avidity induced after vaccination. They also performed multiplex cytokine profiling and intracellular cytokine staining analysis, demonstrating that ChAdOx1 nCoV-19 vaccination induces a predominantly Th1-type response (that appears to be good).
20 December
Editorial. COVID-19 vaccines: the pandemic will not end overnight. The Lancet Microbe December 18, 2020. Full-text: https://doi.org/10.1016/S2666-5247(20)30226-3
See title. Even a global mass immunization program will not immediately end the COVID-19 pandemic. Although control over the infection’s most harmful effects is expected and limiting its spread can be hoped for, it will likely be a few years before the virus can be brought under control worldwide.
19 December
Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med, December 17 2020; 383:2427-2438. Full-text: https://doi.org/10.1056/NEJMoa2028436
Moderna’s messenger RNA vaccine (mRNA-1273) seems to work in older people. In this Phase I, dose-escalation, open label trial in 40 older adults, serum neutralizing activity was detected in all the participants by multiple methods after the second immunization. Solicited adverse events were dose-dependent and predominantly mild or moderate in severity.
17 December
Rid A, Lipsitch M, Miller FG, et al. The Ethics of Continuing Placebo in SARS-CoV-2 Vaccine Trials. JAMA December 14, 2020. Published online December 14, 2020. Full-text: https://doi.org/10.1001/jama.2020.25053
Participants who received placebo in the vaccine trials have made an essential contribution to testing vaccine safety and efficacy. This important viewpoint argues that given limited vaccine supply for at least several months, only the participants receiving placebo who would be eligible for vaccination outside the trial should be offered access to the vaccines at this point. What did the informed consent say? Did it contemplate a EUA or other vaccines getting to market before their specific trial finishes?
Wang W, Wu Q, Yang J, et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ 15 December 2020; 371. Full-text: https://doi.org/10.1136/bmj.m4704
For the record: The adult population willing to be vaccinated is estimated at 3.7 billion (95% confidence interval 3.2 to 4.1 billion).
16 December
Bubar KM, Reinhold K, Kissler SM, et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. medRxiv 2020, posted 7 December. Full-text: https://doi.org/10.1101/2020.09.08.20190629
Over the coming months, the supply of SARS-CoV-2 vaccine will be limited. Who should receive the first available vaccine doses? It depends. If your priority is preventing as many transmissions as possible, give the vaccine to adults aged 20-49. If you want to minimize mortality and years of life lost, vaccine the 60+ years old.
15 December
Oliver S, Gargano J, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. ePub: 13 December 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6950e2
On December 12, 2020, the Advisory Committee on Immunization Practices (ACIP) issued an interim recommendation for use of the Pfizer-BioNTech COVID-19 vaccine in persons aged ≥ 16 years for the prevention of COVID-19. Mass vaccinations in the US started two days later.
14 December
Noval Rivas M, Ebinger JE, Wu M, et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of healthcare workers. J Clin Invest. 2020 Nov 19:145157. PubMed: https://pubmed.gov/33211672. Full-text: https://doi.org/10.1172/JCI145157
A history of BCG vaccination was associated with decreased seroprevalence of anti-SARS-CoV-2 IgG and reduced reported COVID-19-related clinical symptoms in a cohort of healthcare workers in Los Angeles. Of the 6201 HCWs, 29,6% reported a history of BCG vaccination whereas 68,9% did not receive BCG vaccination. Seroprevalence of anti-SARS-CoV-2 IgG as well as incidence of self-reported clinical symptoms associated with COVID-19 were significantly decreased among HCWs with a history of BCG vaccination compared to those without BCG vaccination.
See also the comment by Netea MG, van der Meer JW, van Crevel R. BCG vaccination in healthcare providers and the protection against COVID-19. J Clin Invest. 2020 Dec 11:145545. PubMed: https://pubmed.gov/33306484. Full-text: https://doi.org/10.1172/JCI145545
12 December
Rubin EJ, Longo DL. SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention (Editorial). N Engl J Med 2020, published 10 December. Full-text: https://doi.org/10.1056/NEJMe2034717
Before December 2020, no existing vaccines had been shown to be effective against infection with any beta-coronavirus; strategies to increase the speed of vaccine development had never been tested; and no vaccines based on mRNA technologies had yet been approved. Now, with the paper by Polack et al. that we presented yesterday, all this has been done and the NEJM editors qualify it as a triumph that holds the promise of saving uncounted lives. Rightly, they continue questioning: “Will unexpected safety issues arise when the number grows to millions and possibly billions of people? Will side effects emerge with longer follow-up? Implementing a vaccine that requires two doses is challenging. What happens to the inevitable large number of recipients who miss their second dose? How long will the vaccine remain effective? Does the vaccine prevent asymptomatic disease and limit transmission? And what about the groups of people who were not represented in this trial, such as children, pregnant women, and immunocompromised patients of various sorts?” The story will continue…
Singh JA, Upshur EG. The granting of emergency use designation to COVID-19 candidate vaccines: implications for COVID-19 vaccine trials. Lancet Infect Dis 2020, published 8 December. Full-text: https://doi.org/10.1016/S1473-3099(20)30923-3
In the next weeks, SARS-CoV-2 candidate vaccines will be granted emergency use authorizations after only months of clinical experience. The authors caution that emergency use designations could inadvertently threaten ongoing vaccine research that is yet to define immunological correlates of protection against COVID-19, which could vary according to the vaccine platform, individual characteristics, age groups, and population subset.
11 December
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020, published 10 December. Full-text: https://doi.org/10.1056/NEJMoa2034577
Safety and efficacy findings from the phase 2/3 trial evaluating the safety, immunogenicity, and efficacy of 30 μg of the Pfizer/BioNTech vaccine candidate BNT162b2. A two-dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Reactogenicity was generally mild or moderate, and reactions were less common and milder in older adults than in younger adults. A must-read.
Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med 2020; 383:2320-2332. Full-text: https://doi.org/10.1056/NEJMoa2026920
The first Phase I-II results of NVX-CoV2373 (Novavax), a recombinant nanoparticle vaccine. Cheryl Keech et al. evaluated the safety and immunogenicity of the vaccine in 131 adults using 5-μg and 25-μg doses, with or without Matrix-M1 adjuvant. Reactogenicity was absent or mild in the majority of participants and more common with adjuvant. The addition of adjuvant resulted in enhanced immune responses, was antigen dose–sparing, and induced CD4+ T cell responses that were biased toward a Th1 phenotype. The two-dose 5-μg adjuvant regimen induced geometric mean anti-spike IgG (63,160 ELISA units) and neutralization (3906) responses that exceeded geometric mean responses in convalescent serum from mostly symptomatic COVID-19 patients (8344 and 983, respectively). NVX-CoV2373 is composed of trimeric full-length SARS-CoV-2 spike glycoproteins and a Matrix-M1 adjuvant.
10 December
Voysey M, Clemens SA, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet December 08, 2020. Full-text: https://doi.org/10.1016/S0140-6736(20)32661-1
60-70% protection: this is what we can probably expect from inactivated vaccines. This important paper describes the first interim safety and efficacy data for a viral vector coronavirus vaccine, ChAdOx1 nCoV-19 (AZD1222, developed at Oxford University), evaluated in four trials across three continents. Between April 23 and Nov 4, 2020, 23.848 participants were recruited and vaccinated: 1077 in COV001 (UK), 10.673 in COV002 (UK), 10.002 in COV003 (Brazil), and 2096 in COV005 (South Africa). The vaccine showed significant vaccine efficacy of 70,4% after two doses and protection of 64,1% after at least one standard dose, against symptomatic disease. Across all four studies, the vaccine had a good safety profile with serious adverse events and adverse events of special interest balanced across the study arms. The efficacy of 90,0% seen in those who received a low dose as prime in the UK by error (COV002, read how this dosing error happened and about the implications) was intriguingly high compared with the other findings in the study. However, this has to be confirmed. Moreover, pre-specified sub-group analyses (elderly, those with comorbidities) were not included in this report. Of note, the ChAdOx1 vaccine can be easily administered in existing healthcare systems (in contrast to mRNA vaccines), stored at ‘fridge temperature’ (2-8 °C) and distributed via existing logistics.
Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet December 08, 2020. Full-text: https://doi.org/10.1016/S0140-6736(20)32623-4
In their comment on the ChAdOx1 paper, Maria Deloria Knoll and Chizoba Wonodi are enthusiastic: “Despite the outstanding questions and challenges in delivering these vaccines, it is hard not to be excited about these findings”. They believe that “perhaps by this time next year, we can celebrate the global control of SARS-CoV-2, in person”. We‘ll see.
Pfizer / BioNTech. Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020. FDA submission 2020, published 8 December 2020. PDF: 1) FDA document – 2) Sponsor document
On December 10, Pfizer and its partner, the German company BioNTech, will be publicly reviewed by the FDA. Find here two documents (53 and 92 pages, respectively) which go into the details of the Phase III trial on 44.000 volunteers recruited in the United States, Brazil and Argentina. Among the first 170 infected persons, 162 had received the placebo and 8 the vaccine – an effectiveness of 95%. The documents disclose more details about protection after the first vaccine dose as well as the protection of elderly people, obese people and those suffering from co-morbidities. There seem to have been no significant differences between the vaccinees and the placebo group in the few serious complications recorded. However, moderate side effects were recorded after the second injection: headache, fatigue, muscle pain and chills affected up to 50% of the vaccinees, particularly those under 55 years old.
5 December
Ledford H, Cyranoski D, Van Noorden R. The UK has approved a COVID vaccine — here’s what scientists now want to know. Nature 2020, published 3 December. Full-text: https://www.nature.com/articles/d41586-020-03441-8
The Pfizer–BioNTech vaccine has passed safety and efficacy tests — but scientists still have many questions about how this and other vaccines will perform as they’re rolled out to millions of people.
Danaiya Usher A. South Africa and India push for COVID-19 patents ban. Lancet 2020, published 5 December. Full-text: https://doi.org/10.1016/S0140-6736(20)32581-2
South Africa and India want the World Trade Organization to temporarily suspend intellectual property rights so that COVID-19 vaccines and other new technologies are accessible for poor countries. Right on.
Widge AT, Rouphael NG, Jackson LA. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med 2020, published 3 December. Full-text: Full-text: https://doi.org/10.1056/NEJMc2032195
The correlates of protection against SARS-CoV-2 infection are not yet established. In this short letter, Alicia Widge et al. report the results of immunogenicity studies 3 months after the second vaccination with mRNA-1273. (The 57 days results were published by Jackson et al. in July and Anderson et al. in September.) The data shows that mRNA-1273 produced high levels of binding and neutralizing antibodies that declined slightly over time but they remained elevated in all participants 3 months after the booster vaccination. The authors conclude that mRNA-1273 has the potential to provide durable humoral immunity. Studies of vaccine-induced B cells are ongoing.
Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020. MMWR Morb Mortal Wkly Rep. ePub: 3 December 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6949e1
Over the coming weeks and months, demand for vaccines will exceed supply. In the initial phase of the COVID-19 vaccination program, both health care personnel and residents of long-term care facilities should be offered priority vaccination.
3 December
WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation. Placebo-Controlled Trials of Covid-19 Vaccines — Why We Still Need Them. N Engl J Med 2020, published 2 December. Full-text: https://doi.org/10.1056/NEJMp2033538
In this Perspective, the participants in a World Health Organization ad hoc consultation on the next steps for COVID-19 vaccine evaluation recommend on how to proceed clinically as the first commercial vaccines increasingly become available. Yes, continue with placebo-controlled trials (the bedrock of modern clinical decision-making) because we still need more data on longer-term safety and duration of protection; on whether waning of vaccine-induced protection may lead to vaccine-enhanced disease if a vaccinee becomes infected after exposure to SARS-CoV-2; on information on protection against clinically severe forms of COVID-19; and knowledge of any associations between the degree of protection and the recipient’s age or co-existing conditions. No, refrain from observational studies which are subject to substantial biases and are much less amenable to unambiguous interpretation.
Branswell H. The Covid-19 vaccines are a marvel of science. Here’s how we can make the best use of them. STAT 2020, published 2 December. Full-text: https://www.statnews.com/2020/12/02/how-society-can-make-the-most-of-covid-19-vaccines/
Will 95% efficacy vaccines show the way to a straight road back to Normalville? Or will the route back be a meandering country lane with detours and potholes? Pothole 1: Vaccine skepticism. Pothole 2: Pregnant women. Pothole 3: Children. Pothole 4: How to continue important Phase III randomized trials while vaccines are already on the market? Pothole 5: Understanding SARS-CoV-2 transmission by vaccinated individuals. Have enough time before starting this long-read article.
2 Dezember
Behr MA, Divangahi M, Schurr E. Lessons from BCG for SARS-CoV-2 vaccine candidates. J Infect Dis 2020, published 30 November. Full-text: https://doi.org/10.1093/infdis/jiaa637
A note of caution: according to Marcel Behr and colleagues from Montréal, developers of SARS-CoV-2 vaccines should consider some of the lessons from a ‘new’ vaccine introduced in 1921, where BCG introduced to great fanfare had no measurable effect on the global epidemic, despite evidence of protection at the individual level.
1 Dezember
Sanchez-Felipe L, Vercruysse T, Sharma S et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature December 1, 2020. Full-text: https://doi.org/10.1038/s41586-020-3035-9
Yellow Fever 17D (YF17D) is a small RNA live-attenuated virus with limited vector capacity. The YF17D vaccine is known to rapidly induce broad multi-functional innate, humoral and cell-mediated immune responses that may result in life-long protection following a single vaccine dose in nearly all vaccinees. These favorable characteristics translate also to vectored vaccines based on the YF17D backbone. Consequently, YF17D is used as vector in two licensed human vaccines, generated by swapping genes encoding the YF17D surface antigens for those of Japanese encephalitis or dengue viruses. Here, the authors describe the discovery of a live virus-vectored SARS-CoV-2 vaccine candidate using the YF17D vaccine as vector to express a non-cleavable prefusion form of the SARS-CoV-2 Spike antigen. Safety, immunogenicity and efficacy after a single dose are shown in several animal models such as hamsters, mice and macaques.
30 November
MacPherson A, Hutchinson N, Schneider O, et al. Probability of Success and Timelines for the Development of Vaccines for Emerging and Reemerged Viral Infectious Diseases. Ann Int Med 24 November 2020. Full-text: https://doi.org/10.7326/M20-5350
If a SARS-CoV-2 vaccine is licensed within 18 months of the start of the pandemic, it will mark an unprecedented achievement for non-influenza viral vaccine development. The authors took a look at other vaccines for emerging and re-emerged viral infectious diseases at ClinicalTrials.gov: in total, 606 clinical trials that formed part of 220 distinct development trajectories were identified. The probability of vaccines progressing from Phase II to licensure within 10 years was 10,0%, with most approvals representing H1N1 or H5N1 vaccines. The average timeline from Phase II to approval was 4,4 years. The probabilities of advancing from Phase I to II, Phase II to III, and Phase III to licensure within the total available follow-up time were 38,2%, 38,3%, and 61,1%, respectively.
29 November
Wadman M. Public needs to prep for vaccine side effects. Science 27 November 2020: Vol. 370, Issue 6520, pp. 1022. Full-text: https://doi.org/10.1126/science.370.6520.1022
Expect a rough night after vaccination: A subset of people may face intense, if transient, side effects, called reactogenicity. In this interesting article, Meredith Wadman argues that transparency is key. Rather than minimizing the chance of fever, vaccine administrators could alert people that they may experience a fever that can feel severe but is temporary.
28 November
Editors. The COVID vaccine challenges that lie ahead. Nature. 2020 Nov;587(7835):522. PubMed: https://pubmed.gov/33235368. Full-text: https://doi.org/10.1038/d41586-020-03334-w
Large clinical trials of four vaccine candidates are showing remarkable promise, with three exceeding 90% efficacy — all unexpectedly high. None reported worrying safety signals and one has shown promise in older adults who are particularly vulnerable to SARS-CoV-2 but sometimes respond less well to vaccines. But, there remains a lot of work to do for researchers and clinicians.
Cohen J. After dosing mix-up, latest COVID-19 vaccine success comes with big question mark. Science 2020, published 25 November. Full-text: https://www.sciencemag.org/news/2020/11/after-dosing-mix-latest-covid-19-vaccine-success-comes-big-question-mark
Callaway E. Why Oxford’s positive COVID vaccine results are puzzling scientists. Nature 2020, published 23 November. Full-text: https://www.nature.com/articles/d41586-020-03326-w
Preliminary data from the AstraZeneca vaccine are puzzling. Two full doses given a month apart would be 62% effective, but a half dose followed by a full dose would be 90% effective. Now researchers are trying desperately to instill meaning into these results. Let Jon Cohen and Ewen Callaway explain, sort of.
Editors. Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol 2020, published 27 November. Full-text: https://doi.org/10.1038/s41565-020-00820-0
If approved, BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna/NIH), credited in press releases with sky-rocketing efficacy, would be the first messenger RNA (mRNA)-based vaccines to be used in large populations. mRNA vaccines use nanotechnology platforms to deliver the genetic sequence of specific viral proteins to the host cells. Find more about the founding principles of nanomedicine in this short editorial.
27 November
Wang J. New strategy for COVID-19 vaccination: targeting the receptor-binding domain of the SARS-CoV-2 spike protein. Cell Mol Immunol 2020, published 26 November. Full-text: https://doi.org/10.1038/s41423-020-00584-6
Junzhi Wang comments on a study by Yang J, Wang W, Chen Z et al. [A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, published 29 July. Full-text: https://doi.org/10.1038/s41586-020-2599-8] we presented on 30 July. The authors show that a recombinant spike receptor-binding domain (RBD) protein of SARS-CoV-2 prepared from insect cells could induce a potent functional antibody response in mice, rabbits and non-human primates as early as 7 or 14 days after a single dose injection. Even one dose of the vaccine generated viral neutralizing activity. The vaccine protected non-human primates from live SARS-CoV-2 challenge 28 days after the first vaccination.
25 November
Ma X, Zou F, Yu F, et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity November 25, 2020. Full-text: https://doi.org/10.1016/j.immuni.2020.11.015
A promising new vaccination approach: Xiancai Ma and colleagues from Guangdong, China developed nanoparticle vaccines by covalently conjugating the self-assembled 24-mer ferritin to the receptor binding domain (RBD) and/or heptad repeat (HR) subunits of spike (S) protein. Compared to monomer vaccines, nanoparticle vaccines elicited more robust neutralizing antibodies and cellular immune responses. hACE2 transgenic mice vaccinated with RBD and/or RBD-HR nanoparticles exhibited reduced viral load in the lungs after SARS-CoV-2 challenge. RBD-HR nanoparticle vaccines also promoted neutralizing antibodies and cellular immune responses against other coronaviruses. The nanoparticle vaccination of rhesus macaques induced neutralizing antibodies, and T and B cell responses prior to boost immunization; these responses persisted for longer than three months.
24 November
Sun W, Leist SR, McCroskery S, et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine November 21, 2020. Full-text: https://doi.org/10.1016/j.ebiom.2020.103132
The Newcastle disease virus vector vaccine has some advantages similar to those of other viral vector vaccines. The NDV vector can be amplified in embryonated chicken eggs, which allows for high yields and low costs perdose. Also, the NDV vector is not a human pathogen, therefore the delivery of the foreign antigen would not be compromised by any pre-existing immunity in humans. Weina Sun and colleagues describe NDV vector vaccines expressing the spike protein of SARS-CoV-2 in its wild type format or a membrane-anchored format lacking the polybasic cleavage site. The NDV vector vaccines elicited high levels of antibodies that are neutralizing when the vaccine is given intramuscularly in mice. Importantly, these COVID-19 vaccine candidates protect mice from a mouse-adapted SARS-CoV-2 challenge with no detectable viral titer and viral antigen in the lungs. The results suggested that the NDV vector expressing either the wild type S or membrane-anchored S without the polybasic cleavage site could be used as live vector vaccine against SARS-CoV-2.
McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020. MMWR Morb Mortal Wkly Rep. ePub: 23 November 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6947e3
Four ethical principles will assist the Advisory Committee on Immunization Practices (ACIP) in formulating recommendations for the initial allocation of COVID-19 vaccine: 1) maximizing benefits and minimizing harms; 2) promoting justice; 3) mitigating health inequities; and 4) promoting transparency. Read how application of ethical principles to four candidate groups for initial COVID-19 vaccine allocation is planned in the US.
23 November
Lederer K, Castaño D, Atria DG, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Cell November 21, 2020. Full-text: https://doi.org/10.1016/j.immuni.2020.11.009
A systematic comparison between two vaccine platforms, nucleoside modified mRNA lipid nanoparticle and recombinant protein formulated with the MF59-like adjuvant AddaVax (rRBD-AddaVax), evaluating quantitatively and qualitatively the germinal center (GC) responses to SARS-CoV-2 upon immunization. The authors found that SARS-CoV-2 mRNA vaccines had a superior capacity, in comparison to rRBD-AddaVax, to elicit potent SARS-CoV-2 specific GC B cell responses after the administration of a single vaccine dose. Importantly, they demonstrated that GC B cells and Tfh cells strongly correlated with the production of nAbs.
22 November
Lewis JR. What Is Driving the Decline in People’s Willingness to Take the COVID-19 Vaccine in the United States? JAMA Health Forum. 2020; 1(11):e201393. Full-text: https://doi.org/10.1001/jamahealthforum.2020.1393
People in the US are ready to move on from the COVID-19 pandemic, but when it comes to a vaccine, many have a wait-and-see attitude. Jarrett Ramos Lewis addresses the reasons. As we move toward having an approved COVID-19 vaccine, it is important to understand that for many, it will take time to feel comfortable and confident in getting the vaccine. While the politicization of the vaccine is to blame for some of that delay, the increased reluctance of people to get a COVID-19 vaccine runs much deeper than politics.
20 November
Editorial. COVID-19 vaccines: no time for complacency. Lancet 2020, published 21 November. Full-text: https://doi.org/10.1016/S0140-6736(20)32472-7
“‘Yes. Yes. Yes.’ That was the response of John Bell, Regius Professor of Medicine at the University of Oxford, when asked whether we could be confident that life will be returning to normal by spring.” Of course, we will not return to normal life within 6 months. Let’s lean back and be satisfied that in less than a year, we have characterized a novel illness, sequenced a new viral genome, developed diagnostics, produced treatment protocols, and established the efficacy of drugs and vaccines in randomized controlled trials. There is no hurry. If we can achieve some kind of pre-COVID-19 ‘normalcy’ by 2022, it would be a feat remembered by generations.
19 November
Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, published 18 November. Full-text: https://doi.org/10.1016/S0140-6736(20)32466-1
Phase II results of a single-blind, randomized, controlled trial that describe the safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine in a wide range of participants, including adults aged 70 years and older. The results are encouraging: ChAdOx1 nCoV-19 appears to be better tolerated in older adults than in younger adults and has similar immunogenicity across all age groups after a boost dose.
See also the comment by Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet 2020, published 18 November. Full-text: https://doi.org/10.1016/S0140-6736(20)32481-8
Knipe DM, Levy O, Fitzgerald KA, Mühberger E. Ensuring vaccine safety. Science 2020, published 17 November. Full-text: https://doi.org/10.1126/science.abf0357
Vaccines are among the most successful medical and public health measures ever implemented and prevent ~6 million deaths globally per year. Efficient SARS-CoV-2 vaccines might prevent a similar number of deaths over the coming years. However, caution the authors, the urgent need for COVID-19 vaccines must be balanced with the imperative of ensuring safety and public confidence in vaccines by following the established clinical safety testing protocols throughout vaccine development, including both pre- and post-deployment.
Wadman M. Fever, aches from Pfizer, Moderna jabs aren’t dangerous but may be intense for some. Science 2020, published 18 November. Full-text: https://www.sciencemag.org/news/2020/11/fever-aches-pfizer-moderna-jabs-aren-t-dangerous-may-be-intense-some
Both the BioNTech/Pfizer and the Moderna/NIH mRNA vaccine reached 95% efficacy in clinical trials of tens of thousands of people. The trials revealed no serious safety concerns. We will learn to accept fever and aches as signs that the vaccine works. Even bone and muscle aches and an almost unbearable 38.9°C fever that lasts 12 hours…
18 November
Zhang Y, Zeng G, Pan H. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet November 17, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30843-4
Phase I/II study of an inactivated vaccine candidate against COVID-19. In total, 743 participants at the Suining County of Jiangsu province, China, received at least one dose (n = 143 for Phase 1 and n = 600 for Phase 2; safety population). At day 28 after the days 0 and 28 vaccination schedule, seroconversion of neutralising antibodies was seen for 109 (92%) of 118 participants in the 3 μg group which is the suggested dose for efficacy assessment in future Phase III trials. Adverse events such as mild injection-site pain, occurred in 81 (17%) of 480 vaccine recipients.
Bar-Zeev N, Kochhar S. Expecting the unexpected with COVID-19 vaccines. Lancet November 17, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30870-7
According to this detailed comment, like all Phase II trials, the results must be interpreted with caution until Phase III results are published. Neutralising titers were substantially lower than those seen in 117 convalescent patients who previously had COVID-19 tested in the same laboratory. A demonstration of longevity of response and of empiric protection from this vaccine candidate will be important.
17 November
Callaway E. COVID vaccine excitement builds as Moderna reports third positive result. Nature NEWS November 16, 2020. Full-text: https://doi.org/10.1038/d41586-020-03248-7
Moderna’s vaccine comprises RNA instructions for cells to produce a modified form of the coronavirus spike protein, the immune system’s key target against coronaviruses. Of note, the vaccine remains stable in conventional refrigerators for a month and ordinary freezers for six months. Ewen Callaway summarizes preliminary data showing that the immunization is 94% effective and seems to prevent severe infections.
16 November
Halstead SB, Katzelnick L. COVID-19 Vaccines: Should We Fear ADE? J Infect Dis. 2020 Nov 13;222(12):1946-1950. PubMed: https://pubmed.gov/32785649. Full-text: https://doi.org/10.1093/infdis/jiaa518
Scott B. Halstead and Leah Katzelnick say no. Antibody-dependent enhanced (ADE) breakthrough infections are unlikely because coronavirus diseases in humans lack the clinical, epidemiological, biological, or pathological attributes of ADE disease exemplified by dengue viruses (DENV). In contrast to DENV, SARS and MERS CoVs predominantly infect respiratory epithelium, not macrophages.
12 November
Strassle C, Jardas E, Ochoa J, et al. Covid-19 Vaccine Trials and Incarcerated People — The Ethics of Inclusion. N Engl J Med 2020; 383:1897-1899; published 12 November. Full-text: https://doi.org/10.1056/NEJMp2025955
The most severe COVID-19 outbreaks in the US are no longer occurring in nursing homes or meat packing plants, but in correctional facilities. Find an audio interview (19:26) with Holly Taylor on the ethical issues involved in conducting COVID-19 vaccine research in correctional facilities (19:26).
Teerawattananon Y, Dabak SV. COVID vaccination logistics: five steps to take now. Nature 2020, published 9 November. Full-text: https://www.nature.com/articles/d41586-020-03134-2
The authors point out that creating a safe and effective vaccine is just Act 1 of the 2021 Vaccine Play. Developing a comprehensive and strategic plan for vaccine roll-out is Act 2.
11 November
Walls AC, Fiala B, Schäfer A, et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell. 2020 Oct 31:S0092-8674(20)31450-1. PubMed: https://pubmed.gov/33160446. Full-text: https://doi.org/10.1016/j.cell.2020.10.043
Are self-assembling protein nanoparticles that display 60 SARS-CoV-2 spike receptor-binding domains (RBDs) and induce neutralizing antibody titers comparable to those produced by people after SARS-CoV-2 infection? That’s what Neil King and David Veesler and colleagues from University of Washington, Seattle, US, report. The authors anticipate that manufacture of the nanoparticle vaccines might be very scalable.
McPartlin SO, Morrison J, Rohrig A, Weijer C. Covid-19 vaccines: Should we allow human challenge studies to infect healthy volunteers with SARS-CoV-2? BMJ. 2020 Nov 9;371:m4258. PubMed: https://pubmed.gov/33168564. Full-text: https://doi.org/10.1136/bmj.m4258
The need for COVID-19 vaccines has prompted thousands of otherwise healthy people to volunteer to be infected with the virus to test candidate vaccines. Seán O’Neill McPartlin, Abie Rohrig, and Josh Morrison urge us to embrace the altruism of volunteers, but Charles Weijer argues that it would be dangerous and unjustified.
10 November
Callaway E. What Pfizer’s landmark COVID vaccine results mean for the pandemic. Nature NEWS 09 November 2020. Full-text: https://www.nature.com/articles/d41586-020-03166-8
Yesterday, Pfizer and BioNTech announced that their mRNA-based vaccine candidate, BNT162b2, demonstrated “evidence of efficacy“, based on the first interim efficacy and safety analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee from the Phase III clinical study.
- BNT162b2 was found to be “more than 90% effective” in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection
- Analysis evaluated 94 confirmed cases of COVID-19 in trial participants
- Study enrolled 43,538 participants, with 42% having diverse backgrounds, and no serious safety concerns have been observed
- Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints
That’s what we know. Read how scientists welcome the first compelling evidence that a vaccine can prevent COVID-19. But many questions remain about how much protection it offers, to whom and for how long.
Che Y, Liu X, Pu Y, et al. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clinical Infectious Diseases, 09 November 2020 ciaa1703. Full-text: https://doi.org/10.1093/cid/ciaa1703
In this randomized, double-blinded Phase II trial, 742 healthy adults received a medium (MD) or a high dose (HD) of an inactivated vaccine at an interval of either 14 days or 28 days. Neutralizing antibody (NAb) and anti-S and anti-N antibodies were detected at different times, and adverse reactions were monitored for 28 days after full immunization. The seroconversion rates of NAb in MD and HD groups were 89% and 96% at day 14 and 92% and 96% at day 28 after immunization. Of note, the vaccine was safe (still an issue with inactivated vaccines), and no severe adverse effects were reported.
1 November
Kahn JP, Henry LM, Mastroianni C, et al. Opinion: For now, it’s unethical to use human challenge studies for SARS-CoV-2 vaccine development. PNAS October 29, 2020. Full-text: https://doi.org/10.1073/pnas.2021189117
Important comment: see title. According to the authors, human challenge studies (HCS) to address SARS-CoV-2 face unacceptable ethics challenges, and, further, undertaking them would do a disservice to the public by undermining already strained confidence in the vaccine development process. Ultimately, the social value of these HCS (in terms of deaths averted) hinges on the premise that people at greatest risk of COVID-19-related mortality will receive a safe and efficacious vaccine sooner than they would without HCS. Read why this will be probably not the case and why HCS would do more harm than good.
31 October
Kirby T. COVID-19 human challenge studies in the UK. Lancet October 30, 2020. Full-text: https://doi.org/10.1016/S2213-2600(20)30518-X
Some thoughts about feasibility and ethics of human challenge trials that could potentially accelerate the development of vaccines. The first study phase, which could begin in January 2021, aims to discover the smallest amount of virus it takes to cause the infection in up to 90 healthy young people, aged between 18 and 30 years. The study will probably take place in the high-level isolation unit of the Royal Free Hospital, London, UK. Some commentators have questioned both the timing and the ethical dilemmas presented by the study.
29 October
Schwartz JL. Evaluating and Deploying Covid-19 Vaccines — The Importance of Transparency, Scientific Integrity, and Public Trust. N Engl J Med 2020; 383:1703-1705. Full-text: https://doi.org/10.1056/NEJMp2026393
The situation in the US is dire, public confidence in vaccination is fragile. Jason Schwartz insists that COVID-19 vaccination programs will succeed only if there is widespread belief that available vaccines are safe and effective and that policies for prioritizing their distribution are equitable and evidence-based. He clearly sees that trust in science and expertise are threatened, as the pandemic has shown with catastrophic results. Listen also to the audio interview (12:02).
Lipsitch M, Dean NE. Understanding COVID-19 vaccine efficacy. Science. 2020 Oct 21:eabe5938. PubMed: https://pubmed.gov/33087460. Full-text: https://doi.org/10.1126/science.abe5938
Marc Lipsitch and Natalie Dean publish the shortest abstract in months: “Vaccine efficacy in high-risk groups and reduced viral shedding are important for protection.” Explore strategic prioritization plans.
28 October
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KWR, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2020, published 27 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30773-8
A vaccine against SARS-CoV-2 might act against infection, disease, or transmission and a vaccine capable of reducing any of these elements could contribute to disease control. However, the most important efficacy endpoint, protection against severe disease and death, is difficult to assess in Phase III clinical trials. In this review, Susanne Hodgson and colleagues explore the challenges in assessing the efficacy of candidate SARS-CoV-2 vaccines, discuss the caveats needed to interpret reported efficacy endpoints, and provide insight into answering the seemingly simple question, “Does this COVID-19 vaccine work?” Remember: the fundamental understanding of the pathogen is still evolving.
27 October
Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. PubMed: https://pubmed.gov/32967006. Full-text: https://doi.org/10.1038/s41586-020-2798-3
Brilliant review of SARS-CoV-2 vaccines: vaccine platforms, results from studies on non-human primates and results from Phase I/II trials in humans. Read the review this evening and read it again next week.
Hensel J, McAndrews KM, McGrail DJ, et al. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep 10, 18377 (2020). Full-text: https://doi.org/10.1038/s41598-020-75491-x
Preliminary epidemiological analyses suggested that BCG vaccination might be associated with reduced COVID-19 cases and mortality. Might the BCG vaccine provide protection against infection with SARS CoV-2? (Seventeen clinical trials are currently registered to investigate the potential benefits of BCG vaccinations on exposure to CoV-2.) Now, Raghu Kalluri, Janine Hensel and colleagues challenge this assumption. After correction for confounding variables, most notably testing rates, they found no association between BCG vaccination policy and COVD-19 spread rate or percent mortality.
24 October
Mehrotra DV, Janes HE, Fleming TR, et al. Clinical Endpoints for Evaluating Efficacy in COVID-19 Vaccine Trials. Ann Int Med October 21, 2020. Full-text: https://doi.org/10.7326/M20-6169
Guidance from the FDA recommends minimal Phase III success criteria for approval of a vaccine: an estimated reduction in the primary endpoint of at least 50% in the vaccine group versus the placebo group, with the 95% CI providing assurance of at least a 30% reduction. The FDA guidance also indicates that acceptable primary endpoints for approval could include SARS-CoV-2 infection, symptomatic infection, severe COVID-19, or some combination of these. Biostatistician Devan V. Mehrotra and colleagues emphasize the need to facilitate harmonized evaluation and comparison of the efficacy of these vaccines. They propose a standard set of clinical endpoints to support pooling data for analyses of immunologic surrogate endpoints.
23 October
Bell BP, Romero JR, Lee GM. Scientific and Ethical Principles Underlying Recommendations from the Advisory Committee on Immunization Practices for COVID-19 Vaccination Implementation. JAMA. 2020 Oct 22. PubMed: https://pubmed.gov/33090194. Full-text: https://doi.org/10.1001/jama.2020.20847
Discover how the US wants to distribute a vaccine. This viewpoint discusses possible prioritization scenarios. “Phase Ia” includes health care personnel who have the potential for direct or indirect exposure to patients or infectious materials. This group comprises an estimated 20 million (!) people. We will later come back to Phase Ib (and the rest) maybe in 2022.
Kreps S, Prasad S, Brownstein JS. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw Open. 2020;3(10):e2025594. Full-text: https://doi.org/10.1001/jamanetworkopen.2020.25594
But who will accept such a vaccine (see previous paper). And when? Sarah Kreps and colleagues asked 1971 US adults, analyzing factors associated with willingness and individual preferences. Some interesting findings: The marginal mean willingness to receive a vaccine was lowest when the vaccine was recommended by President Trump. Willingness was slightly (but not significantly) higher with former Vice President Biden and significantly higher given a CDC or WHO endorsement. Respondents who indicated Democratic political partisanship were significantly more likely to report willingness than those who indicated Republican political partisanship. A vaccine originating in China was associated with a 10% lower willingness.
21 October
Bangaru S, Ozorowski G, Turner HL, et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, published 20 October. Full-text: https://doi.org/10.1126/science.abe1502
Andrew Ward, Sandhya Bangaru and colleagues describe the structure of a leading SARS-CoV-2 S vaccine candidate (NVAX-CoV2373, under development by Novavax Inc. and Novavax AB, Uppsala) based on a full-length S, residues 1-1273 which includes the transmembrane (TM) and the cytoplasmic tail (CT). The authors found that NVAX-CoV2372 is stable, homogeneous, and locked in the antigenically preferred pre-fusion conformation. After structural, biophysical, and antigenic characterization, the candidate vaccine will not face the true proof-of-principle: evaluation in humans.
17 October
Xia S, Zhang Y, Wang Y, e al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2020, published 15 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30831-8
A Chinese candidate vaccine, BBIBP-CorV (Beijing Institute of Biological Products), based on inactivated coronavirus, seems to be safe and elicits an antibody response. This is the first study of an inactivated SARS-CoV-2 vaccine to include participants older than 60 years. In these participants, antibodies took up to 42 days to be detected, compared with 28 days for participants aged 18 to 59. As expected, antibody levels were lower in those aged 60 to 80 years. Two-dose immunization with 4 μg vaccine on days 0 and 21 or days 0 and 28 achieved higher neutralizing antibody titers than the single 8 μg dose or 4 μg dose on days 0 and 14. A Phase III trial of BBIBP-CorV is currently underway in Abu Dhabi and the United Arab Emirates.
See also the comment by Isakova-Sivak I, Rudenko L. A promising inactivated whole-virion SARS-CoV-2 vaccine. Lancet Infect Dis 2020, published 15 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30832-X
Krause PR, Grubner MF. Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. N Engl J Med 2020, published 16 October. Full-text: https://doi.org/10.1056/NEJMp2031373
There should be no emergency use authorization (EUA) of any COVID-19 vaccine without a median follow-up duration of at least 2 months after completion of the full phase 3 vaccination regimen. Normally, the FDA requires at least 6 months of safety follow-up for serious and other medically attended adverse events in a sufficient number of vaccinees. Philip Krause and Marion Gruber warn that any curtailment of this minimum follow-up could destroy the scientific credibility for future vaccines in the United States. Also see FDA’s Vaccines and Related Biological Products Committee Open Hearing, 22 Oct 2020, https://www.youtube.com/watch?v=1XTiL9rUpkg&feature=youtu.be.
Kurup D, Wirblich C, Ramage H, et al. Rabies virus-based COVID-19 vaccine CORAVAX™ induces high levels of neutralizing antibodies against SARS-CoV-2. npj Vaccines 5, 98 (2020). Full-text: https://doi.org/10.1038/s41541-020-00248-6
The authors show the rapid development of a novel, efficient, and safe COVID-19 vaccine using a rabies virus-based vector. Both a live and an inactivated rabies virus containing the SARS-CoV-2 spike S1 protein induces potent virus-neutralizing antibodies at much higher levels than seen in the sera of convalescent patients.
15 October
Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med 2020, published 15 October. Full-text: https://www.nejm.org/doi/full/10.1056/NEJMoa2027906?query=featured_home
Safety and immunogenicity data from a phase 1 trial of RNA-based Pfizer/BioNTech vaccines. In both younger (18 to 55 years of age) and older adults (65 to 85 years of age), the two vaccine candidates elicited similar dose-dependent SARS-CoV-2–neutralizing geometric mean titers, comparable or higher than the geometric mean titer of a panel of SARS-CoV-2 convalescent serum samples. The data presented here by Judith Absalon, Edward Walsh and colleagues include those that guided the companies’ decision to advance BNT162b2 at the 30-μg dose level to the phase 2–3, international trial to evaluate its safety and efficacy in participants 18 to 85 years of age.
13 October
Dong Y, Dai T, Wei Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Sig Transduct Target Ther 5, 237 (2020). Full-text: https://doi.org/10.1038/s41392-020-00352-y
The 11-page review for your next weekend. The authors provide an overview of the experimental and clinical data obtained from recent SARS-CoV-2 vaccines trials, and highlight certain potential safety issues that require consideration when developing vaccines. Learn more about antigen design, important and unimportant epitopes, structure design, suitable delivery system and adjuvants.
8 October
McAuley AJ, Kuiper MJ, Durr PA, et al. Experimental and in silico evidence suggests vaccines are unlikely to be affected by D614G mutation in SARS-CoV-2 spike protein. npj Vaccines 5, 96 (2020). https://doi.org/10.1038/s41541-020-00246-8
The D614G mutation of the SARS-CoV-2 spike protein has been speculated to adversely affect the efficacy of vaccines. In this article, S. Vasan, Alexander McAuley and colleagues claim that there is no experimental evidence to support this speculation. They performed virus neutralization assays using sera from ferrets that received two doses of the INO-4800 COVID-19 vaccine, and Australian virus isolates (VIC01, SA01 and VIC31) which either possess or lack this mutation.
29 September
Helfland BK, Webb M, Gartaganis SL, et al. The Exclusion of Older Persons From Vaccine and Treatment Trials for Coronavirus Disease 2019—Missing the Target. JAMA Intern Med, September 28, 2020. Full-text: https://doi.org/10.1001/jamainternmed.2020.5084
Those most in need are excluded: in this important review, Benjamin Helfland and colleagues analyzed clinical COVID-19 trials for age exclusions. In 232 Phase 3 clinical trials, 38 included age cut-offs and 77 had exclusions preferentially affecting older adults. Of 18 vaccine trials, 11 included age cut-offs, and the remaining 7 had broad non-specified exclusions. These findings indicate that older adults are likely to be excluded from more than 50% of COVID-19 clinical trials and 100% of vaccine trials. Why? Such exclusion will limit the ability to evaluate the efficacy, dosage, and adverse effects of the intended treatments.
28 September
Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. npj Vaccines 5, 91 (2020). Full-text: https://doi.org/10.1038/s41541-020-00243-x
Hanneke Schuitemaker, Rinke Bos and colleagues report more details about Ad26.COV2.S which is currently being evaluated in a clinical trial (ClinicalTrials.gov: NCT04436276). Vaccines based on transgenes delivered by recombinant replication-incompetent adenovirus type 26 vectors (Ad26) have previously been shown to have an acceptable safety profile in humans and are able to induce neutralizing and binding antibodies, CD4 and CD8 T cell responses and a Th1-biased immune response in animals and humans.
26 September
Cookson C. UK to test vaccines on volunteers deliberately infected with Covid-19. Financial Times 2020, published 23 September. Full-text: https://www.ft.com/content/b782f666-6847-4487-986c-56d3f5e46c0b
In the world’s first COVID-19 ‘human challenge trials’ healthy volunteers will be deliberately infected with SARS-CoV-2 to assess the effectiveness of experimental vaccines. See also the CR Top 10, June 4: Jamrozik E, Selgelid MJ. COVID-19 human challenge studies: ethical issues. Lancet Infect Dis. 2020 May 29:S1473-3099(20)30438-2. PubMed: https://pubmed.gov/32479747. Full-text: https://doi.org/10.1016/S1473-3099(20)30438-2
Human challenge studies could accelerate vaccine development, helping to test multiple candidate vaccines. This personal view on ethical issues explains why this will be difficult. The authors argue that human challenge studies can “reasonably be considered ethically acceptable insofar as such studies are accepted internationally and by the communities in which they are done, can realistically be expected to accelerate or improve vaccine development, have considerable potential to directly benefit participants, are designed to limit and minimise risks to participants, and are done with strict infection control measures to limit and reduce third-party risks.”
25 September
Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020 Sep 3:S0140-6736(20)31866-3. PubMed: https://pubmed.gov/32896291. Full-text: https://doi.org/10.1016/S0140-6736(20)31866-3
On September 5, we commented that it was high time to see some data on an “approved” vaccine, consisting of two recombinant adenovirus vectors carrying the spike glycoprotein (Sputnik V, presented as the world’s “premiere”, like planting a tiny flag in the sea bed two and a half miles beneath the North Pole in 2007).
Bucci E, Andreev, Björkman A, et al. Safety and efficacy of the Russian COVID-19 vaccine: more information needed. Lancet September 21, 2020. Full-text: https://doi.org/10.1016/S0140-6736(20)31960-7
A few days later, the study received these notes of serious concerns. Dozens of authors raised doubts about the reliability of the data. The main issue (among many others): there were several data patterns which appeared repeatedly for the reported experiments. A Photoshop fake? Enrico Bucci and colleagues conclude that “in lack of the original numerical data, no conclusions can be definitively drawn on the reliability of the data presented, especially regarding the apparent duplications detected”. For more details see also https://cattiviscienziati.com/2020/09/07/note-of-concern/
Logunov DY, Dolzhikova IV, Tukhvatullin AI. Safety and efficacy of the Russian COVID-19 vaccine: more information needed – Authors’ reply. Lancet September 21, 2020. Full-text: https://doi.org/10.1016/S0140-6736(20)31970-X
The author’s reply. They “confirm that individual participant data will be made available on request to DYL and that after approval of a proposal, data can be shared through a secure online platform”. Shall we hold our breath?
10 September
Abbasi J. COVID-19 and mRNA Vaccines-First Large Test for a New Approach. JAMA. 2020 Sep 3. PubMed: https://pubmed.gov/32880613. Full-text: https://doi.org/10.1001/jama.2020.16866
mRNA vaccines like BNT162b2 from BioNTech and Pfizer and mRNA-1273 by Moderna have ‘the potential to be truly transformative’ (Drew Weissman) but have never been tested in large-scale human trials. Now both vaccines have entered Phase 3 trials, which together will enroll an estimated 60,000 volunteers. Follow Jennifer Abbasi on a tour of ‘proof in the pudding’ and mRNA vaccines beyond COVID-19.
9 September
Phillips N, Cyranoski D, Mallapathy S. A leading coronavirus vaccine trial is on hold: scientists react. Nature News September 9, 2020. Full-text: https://www.nature.com/articles/d41586-020-02594-w
This article summarizes what is known about the news of the day: AstraZeneca has reported a case of a transverse myelitis in a person who received AZD1222, an adenoviral-vector vaccine that harnesses a cold-causing ‘adenovirus’ isolated from chimpanzees. The Phase III trial was “voluntarily paused”. However, details of the adverse event, including how serious it was and when it happened, have not been reported. It is still unclear whether the person received the vaccine or placebo. Let’s wait for the details.
Shah S, Patel J, Alchaki AR. Development of Transverse Myelitis after Vaccination. A CDC/FDA Vaccine Adverse Event Reporting System (VAERS) Study, 1985–2017. Neurology April 10, 2018; 90. Abstract: https://n.neurology.org/content/90/15_Supplement/P5.099
In the meantime, you may read this review of 119 cases of transverse myelitis (TM) occurring after vaccination, reported during a period of over 30 years to the FDA. Although the reporting rate of post-vaccination TM was in the range expected in the general population, the unbalanced distribution of these cases in the first 6 weeks after vaccination suggested that the association between vaccination and some cases may not be coincidental. (For antivaxxers: this is rare!)
8 September
Jeyanathan M, Afkhami S, Smaill F, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 2020, published 4 September. Full-text: https://doi.org/10.1038/s41577-020-00434-6
In this review, Zhou Xing, Mangalakumari Jeyanathan and colleagues describe the immunological principles of SARS-CoV-2 vaccine development and analyze the current vaccine candidates, their strengths and potential shortfalls. They also make inferences about their chances of success. A hazardous undertaking.
6 September
Tostanoski LH, Wegmann F, Martinot AJ, et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med. 2020 Sep 3. PubMed: https://pubmed.gov/32884153. Full-text: https://doi.org/10.1038/s41591-020-1070-6
It’s not only protection from infection but also from severe disease. In hamsters, a single immunization with an adenovirus serotype 26 vector-based vaccine expressing a stabilized SARS-CoV-2 spike protein elicited binding and neutralizing antibody responses and protected against weight loss, pneumonia and mortality.
5 September
Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, published 4 September. Full-text: https://doi.org/10.1016/S0140-6736(20)31866-3
It was high time to see some data on an “approved” vaccine. See also the comment by Naor Bar-Zeev and Tom Inglesby [Bar-Zeev N, Inglesby T. COVID-19 vaccines: early success and remaining challenges. Lancet 2020, published 4 September. Full-text: https://doi.org/10.1016/S0140-6736(20)31867-5].
Fisher KA, Bloomstone SJ, Walter J, et al. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann Intern Med 2020, published 4 September. Full-text: https://doi.org/10.7326/M20-3569
In a few months, when we have a vaccine, will people get vaccinated? In a study of 991 participants, Kimberly Fisher and colleagues found that 57.6% of (n = 571) intended to be vaccinated, 31.6% (n = 313) were not sure, and 10.8% (n = 107) did not intend to be vaccinated. Factors independently associated with vaccine hesitancy (a response of “no” or “not sure”) included younger age, Black race, lower educational attainment, and not having received the influenza vaccine in the prior year. The authors conclude that targeted and multipronged efforts will be needed to increase acceptance of a COVID-19 vaccine.
3 September
Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. NEJM September 2, 2020, Full-text: https://doi.org/10.1056/NEJMoa2026920
NVX-CoV2373 is a recombinant SARS-CoV-2 nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant. In 83 participants younger than 60 years of age, two injections of NVX-CoV2373 delivered in the deltoid muscle on day 0 and 21 appeared to be safe. Immune responses exceeded levels in COVID-19 convalescent serum, showing high neutralizing antibody responses and T cells with a predominant Th1 phenotype. Phase 2 has started.
2 September
Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S. ACTIVATE: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 2020, published 31 August. Full-text: https://doi.org/10.1016/j.cell.2020.08.051
In this double-blind, randomized trial, 198 elderly patients received BCG or placebo vaccine at hospital discharge and were followed for 12 months. At interim analysis (78 patients allocated to placebo vaccination and 72 patients allocated to BCG vaccination), Evangelos Giamarellos-Bourboulis et al. found that BCG vaccination significantly increased the time to first infection (median 16 weeks compared to 11 weeks after placebo). The incidence of new infections was 42.3% after placebo vaccination and 25.0% after BCG vaccination; most of the protection was against respiratory tract infections of probable viral origin. Any effect on SARS-CoV-2 infection? The number of individuals participating in the trial was too low to allow for any conclusions. Larger trials will provide the answer.
27 August
Slaoui M, Hepburn M. Developing Safe and Effective Covid Vaccines — Operation Warp Speed’s Strategy and Approach. N Engl J Med 2020, published 26 August. Full-text: https://doi.org/10.1056/NEJMp2027405
What is OWS and what does it do? Moncef Slaoui and Matthew Hepburn from Operation Warp Speed explain the forces behind a national vaccine strategy. The players: Pfizer and BioNTech, AstraZeneca and Oxford University, Janssen, Moderna, Janssen, Novavax, Sanofi/GSK. Will they succeed in this unprecedented endeavor?
25 August
Price WN 2nd, Rai AK, Minssen T. Knowledge transfer for large-scale vaccine manufacturing. Science. 2020 Aug 21;369(6506):912-914. PubMed: https://pubmed.gov/32792464. Full-text: https://doi.org/10.1126/science.abc9588
Identifying an effective SARS-CoV-2 vaccine and prove its safety in huge clinical trials is only the first step. The next step is not less challenging: manufacturing vaccines at enormous scale. In this Policy Forum, law school scholars Nicholson Price, Arti Rai and Timo Minssen explain that fast manufacturing will require not only physical capacity but also access to knowledge not contained in patents or in other public disclosures. Follow the authors on a path through the jungle of licenses, know-how transfer, hostage taking and manufacturing secrecy, and discover why large biopharmaceutical firms are now willing to share information that they might previously have viewed as providing competitive advantage.
Callaway E. The unequal scramble for coronavirus vaccines — by the numbers. Nature 2020, published 24 August. Full-text: https://www.nature.com/articles/d41586-020-02450-x
Will SARS-CoV-2 vaccines be only for the rich? Ellen Callaway shows how wealthy countries have struck deals to buy more than two billion doses of coronavirus vaccine. Find out that the UK is the world’s highest per-capita buyer, with 340 million purchased: around 5 doses for each citizen. And read more about COVAX, spearheaded by GAVI, a Geneva-based funder of vaccines for low-income countries, along with CEPI and the World Health Organization. It aims to secure 2 billion vaccine doses. One billion are for 92 low- and middle-income countries and economies (LMICS), which encompass half the world’s population.
23 August
Feng L, Wang Q, Shan C, et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun 11, 4207 (2020). Full-text: https://doi.org/10.1038/s41467-020-18077-5
Ling Chen, Liqiang Feng and colleagues report the generation of a replication-incompetent recombinant serotype 5 adenovirus, Ad5-S-nb2, which elicited systemic S-specific antibody and cell-mediated immune (CMI) responses in mice and rhesus macaques both after intramuscular injection and intranasal inoculation. At 30 days after a single vaccination with Ad5-S-nb2, macaques were protected against SARS-CoV-2 challenge.
20 August
Hassan AP, Kafai NM, Dmitriev IP. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell August 19, 2020. Full-text: https://doi.org/10.1016/j.cell.2020.08.026
In their animal experiments on mice expressing the ACE receptor, Ahmed Hassan and colleagues from St. Louis, US show the protective activity of a chimpanzee adenovirus-vectored vaccine encoding a pre-fusion stabilized Spike protein. Of note, intramuscular dosing induced robust systemic humoral and cell-mediated immune responses but did not confer sterilizing immunity. In contrast, a single intranasal dose induced high levels of neutralizing antibodies, promoted systemic and mucosal IgA and T cell responses, and virtually completely prevented SARS-CoV-2 infection in both the upper and lower respiratory tracts.
15 August
Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020 Aug 13:e2015543. PubMed: https://pubmed.gov/32789505. Full-text: https://doi.org/10.1001/jama.2020.15543
An Pan, Xiaoming Yang and colleagues provide the first interim safety, tolerability, and immune response results for a β-propiolactone–inactivated whole-virus vaccine adjuvanted in 0.5 mg of aluminum hydroxide. The incidence rate of adverse reactions in the current study (15.0% among all participants) was not significantly different between the vaccine groups and the active control (alum) groups; it was also lower compared with results of other SARS-CoV-2 candidate vaccines. The neutralizing antibody response suggested that the inactivated vaccine may effectively induce antibody production, but the optimal interval between injections and times of booster injections of the inactivated vaccine remains unclear. In the discussion, find more about ADE and VAERD. See also the comment by Mark Mulligan: An Inactivated Virus Candidate Vaccine to Prevent COVID-19. JAMA. 2020 Aug 13. PubMed: https://pubmed.gov/32789500. Full-text: https://doi.org/10.1001/jama.2020.15539
12 August
Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, published 12 August. Full-text: https://doi.org/10.1038/s41586-020-2639-4
Mark Mulligan, Kirsten Lyke, Nicholas Kitchin, Judith Absalon and colleagues report the safety, tolerability, and immunogenicity data from an ongoing study among 45 healthy adults, randomized to receive 2 doses, separated by 21 days, of 10 µg, 30 µg, or 100 µg of BNT162b1. BNT162b1, developed by BioNTech and Pfizer, is a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine that encodes trimerized SARS-CoV-2 spike glycoprotein receptor-binding domain (RBD). A clear dose-level response in elicited neutralizing titers was observed after doses 1 and 2 with a particularly steep dose response between the 10 μg and 30 μg dose levels. Geometric mean neutralizing titers reached 1.9- to 4.6-fold that of a panel of COVID-19 convalescent human sera at least 14 days after a positive SARS-CoV-2 PCR. The clinical testing of BNT162b1 is taking place in the context of a broader, ongoing COVID-19 vaccine development program by both companies. That program includes the clinical testing of three additional vaccine candidates, including candidates encoding the full-length spike.
11 August
Dagotto G, Yu J, Barouch DH. Approaches and Challenges in SARS-CoV-2 Vaccine Development. Cell Host Microbe 2020, published 10 August. Full-text: https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(20)30455-8
Progress in SARS-CoV-2 vaccine development to date has been faster than for any other pathogen in history. In this perspective, Dan Barouch, Gabriel Dagotto and Jingyou Yu discuss three topics that are critical for SARS-CoV-2 vaccine development:
- Antigen selection and engineering
- Pre-clinical challenge studies in non-human primate models
- Immune correlates of protection
6 August
Corbett KS, Edwards DK, Leist SR et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, published 5 August. Full-text: https://doi.org/10.1038/s41586-020-2622-0
Barney Graham, Andrea Carfi and colleagues show that mRNA-1273, a vaccine currently tested in Phase 3 trials, protects mice against SARS-CoV-2 infection in the lungs and noses without evidence of immunopathology. The vaccine induced both potent neutralizing antibody responses to wild-type (D614) and D614G mutant2 SARS-CoV-2 and CD8 T cell responses. The authors are prolific – a week ago, they evaluated the same vaccine in non-human primates and published their paper in the N Engl J Med (see Corbett et al., Top 10 July 29). Read also the last paragraph of this week’s paper where Corbett et al. describe a new paradigm for rapid vaccine development.
See also a Nat Biomed Eng editorial: Fast-and-fit vaccines. Published 10 August 2020. Full-text: https://doi.org/10.1038/s41551-020-00605-9
2 August
Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 2020 Jul 30:eabc4730. PubMed: https://pubmed.gov/32732280. Full-text: https://doi.org/10.1126/science.abc4730
First, adapt a clinical isolate of SARS-CoV-2 by serial passaging in the respiratory tract of aged BALB/c mice. When the mouse-adapted strain shows increased infectivity in mouse lung after 6 passages, leading to interstitial pneumonia and inflammatory responses following intranasal inoculation, sequence the virus genome and look for adaptive mutations which might be associated with the increased virulence. That’s what Yusen Zhou, Cheng-Feng Qin, Shihui Sun, Shibo Jiang and colleagues did. They found an N501Y mutation located at the receptor binding domain (RBD) of the spike protein. They also showed the protective efficacy of a recombinant RBD vaccine candidate. They conclude that this MASCp6 could be of value in evaluating vaccines and antivirals against SARS-CoV-2.
30 July
van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, published 30 July. Full-text: https://doi.org/10.1038/s41586-020-2608-y
The good news first or the bad news first? OK, the good news: Vincent Munster, Sarah Gilbert and colleagues showed that vaccination with the adenovirus-vectored ChAdOx1 vaccine (see the July 20 Top 10) induced a balanced Th1/Th2 humoral and cellular immune response in rhesus macaques. The authors observed a significantly reduced viral load in bronchoalveolar lavage fluid and lower respiratory tract tissue, and no pneumonia was observed in vaccinated animals. The bad news (for prevention policies in general and for anti-vaxxers in particular): there was no difference in nasal shedding between vaccinated and control animals. Back to the good news: there was no evidence of immune-enhanced disease following viral challenge in vaccinated animals.
Mercado NB, Zahn R, Wegmann F et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, published 30 July. Full-text: https://doi.org/10.1038/s41586-020-2607-z
For global deployment and pandemic control, a vaccine that requires only a single immunization would be optimal. Hanneke Schuitemaker, Dan Barouch and colleagues developed a series of adenovirus serotype 26 (Ad26) vectors encoding different variants of the SARS-CoV-2 spike (S) protein and showed the immunogenicity and protective efficacy of a single dose of Ad26 vector-based vaccines in 52 rhesus macaques. The optimal Ad26 vaccine induced robust neutralizing antibody responses and provided complete or near-complete protection in bronchoalveolar lavage and nasal swabs following SARS-CoV-2 challenge.
Yang J, Wang W, Chen Z et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, published 29 July. Full-text: https://doi.org/10.1038/s41586-020-2599-8
The authors show that a recombinant vaccine comprising residues 319-545 of the Spike protein receptor-binding domain (S-RBD) can induce a potent functional antibody response in mice, rabbits and non-human primates as early as 7 or 14 days after a single dose injection. Antibodies shared common binding epitopes from infected patients, neutralizing activity was strong, and a simple adjuvant like Alum could further enhance the immune response. Even one dose of the vaccine generated viral neutralizing activity. The vaccine protected non-human primates from live SARS-CoV-2 challenge 28 days after the first vaccination.
Rubin EJ, Baden LR, Morrissey S. New SARS-CoV-2 Vaccine Results. N Engl J Med 2020; 383:e57. Access: https://www.nejm.org/doi/full/10.1056/NEJMe2026514
Audio interview (19:56) with Peter Piot who talks about his own experience with COVID-19, as well as recent developments in SARS-CoV-2 vaccines.
29 July
Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med 2020, published 28 July. Full-text: https://doi.org/10.1056/NEJMoa2024671
Vaccination of non-human primates with mRNA-1273 induces robust SARS-CoV-2 neutralizing activity, rapid protection in the upper and lower airways, and no pathologic changes in the lung. For this important vaccine trial, Barney S. Graham, Robert A. Seder and colleagues divided 12 female and 12 male Indian-origin rhesus macaques into groups of three and vaccinated them intramuscularly at week 0 and at week 4 with either 10 or 100 μg of mRNA-1273 or placebo. At week 8 (4 weeks after the second vaccination), all animals were challenged with SARS-CoV-2. mRNA-1273 induced antibody levels exceeding those found in human convalescent phase serum. Vaccination also induced type 1 helper T cell (Th1)–biased CD4 T cell responses and low or undetectable Th2 or CD8 T cell responses.
No viral replication was detectable in the nose of any of the eight animals in the 100 μg dose group by day 2 after challenge (8 weeks after the first vaccination). The ability to limit viral replication in both the lower and the upper airways will have important implications for vaccine-induced prevention of both SARS-CoV-2 disease and transmission.
Liu G, Carter B, Bricken T, Jain S, Viard M, Carrington M, Gifford DK. Computationally Optimized SARS-CoV-2 MHC Class I and II Vaccine Formulations Predicted to Target Human Haplotype Distributions. Cell Systems 2020, published 27 July. Full-text: https://www.cell.com/cell-systems/fulltext/S2405-4712(20)30238-6
Do you want to optimize peptide vaccine formulations for SARS-CoV-2? David K. Gifford and colleagues from MIT now give you a combinatorial machine learning method. They also encourage the early publication of vaccine designs to enable collaboration and rapid progress toward safe and effective vaccines for COVID-19. Consequently, they provide an open-source implementation of their design methods (OptiVax), vaccine evaluation tool (EvalVax), as well as the data used in their design efforts: https://github.com/gifford-lab/optivax.
28 July
Graham SP, McLean RK, Spencer AJ et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. npj Vaccines 5, 69 (2020). Full-text: https://doi.org/10.1038/s41541-020-00221-3
Simon P. Graham, Teresa Lambe and colleagues compare the immunogenicity of one or two doses of ChAdOx1 nCoV-19 in both mice and pigs. Whilst a single dose induced antigen-specific antibody and T cell responses, a booster immunization enhanced antibody responses, particularly in pigs, with a significant increase in SARS-CoV-2 neutralizing titers. See also the ChAdOx1 Phase 1/2 randomized trial of a chimpanzee adenovirus-vector vaccine (nCoV-19) published a week ago: https://covidreference.com/top-10-july-20.
Martin C, Lowery D. mRNA vaccines: intellectual property landscape. Nat Rev Drug Discov 2020, 27 July. Full-text: https://www.nature.com/articles/d41573-020-00119-8
Cecilia Martin and Drew Lowery generate an intellectual property landscape surrounding mRNA vaccine development. Overall filing activity aims at protecting methods to improve mRNA delivery efficiency as well as pharmacological modifications to reduce mRNA instability and innate immunogenicity. Moderna, CureVac, BioNTech and GSK own nearly half of the mRNA vaccine patent applications.
27 July
Zhang NN, Li XF, Deng YQ. A thermostable mRNA vaccine against COVID-19. Cell 2020, ublished: July 23. Abstract: https://www.cell.com/cell/fulltext/S0092-8674(20)30932-6. Full-text: https://doi.org/10.1016/j.cell.2020.07.024
The authors describe the development of an LNP-encapsulated mRNA vaccine (termed “ARCoV”) which targets the RBD of SARS-CoV-2. The vaccine induces neutralizing antibodies and T cell immunity in mice and non-human primates. Two doses of ARCoV immunization in mice conferred complete protection against the challenge of a SARS-CoV-2 mouse adapted strain. Phase 1.
20 July
Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet, 20 July 2020. Full-text: https://www.thelancet.com/lancet/article/s0140-6736(20)31604-4
Andrew Pollard and colleagues report their Phase 1/2 randomized trial of a chimpanzee adenovirus-vector vaccine (ChAdOx1 nCoV-19) expressing the SARS-CoV-2 spike protein. Study participants received either ChAdOx1 nCoV-19 (n = 543) or a meningococcal conjugate vaccine (MenACWY) as control (n = 534). In ChAdOx1 vaccinees, T cell responses peaked on day 14, anti-spike IgG responses rose by day 28, and neutralizing antibody responses against SARS-CoV-2 were detected in > 90% (find more details in the paper, especially about results after a booster dose). Adverse events such as fatigue, headache, and local tenderness commonly occurred. There were no serious adverse events.
Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 20 July 2020. Full-text: https://www.thelancet.com/lancet/article/s0140-6736(20)31605-6
Wei Chen and colleagues report results from a randomized Phase 2 trial of an Ad5-vector COVID-19 vaccine from a single center in Wuhan. More than 90% of participants had T cell responses, seroconversion of binding antibody occurred in more than 96%, and neutralizing antibodies were seen in about 85%. The authors found that compared with the younger population, older people had a significantly lower immune response, but higher tolerability, to the Ad5-vector COVID-19 vaccine. In a Phase 2b trial, an additional dose might therefore be needed to induce a better immune response in the older population. Adverse events such as fever, fatigue, headache, or local site pain were comparable to the ChAdOx1 study above.
Bar-Zeev N, Moss WJ. Encouraging results from phase 1/2 COVID-19 vaccine trials. Lancet, 20 July 2020. Full-text: https://www.thelancet.com/lancet/article/s0140-6736(20)31611-1
A comment on the two papers above as well as a list of questions to be addressed by the coming Phase 3 trials:
- Will a single dose be sufficient in older adults, or is a booster dose required?
- Does longevity of response or rates of waning differ with a two-dose regimen, and does longevity of clinical protection require cell-mediated responses?
- Are there host-specific differences in immunogenicity by age, sex, or ethnicity?
- Do T cell responses correlate with protection irrespective of humoral titers?
- Are there specific adverse events in pregnant women?
16 July
Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020 Jul 14. PubMed: https://pubmed.gov/32663912. Full-text: https://doi.org/10.1056/NEJMoa2022483
This study conducted in Washington and Atlanta evaluated the candidate vaccine mRNA-1273 that encodes the stabilized prefusion SARS-CoV-2 spike protein. In a Phase I open label trial, 45 healthy adults received two vaccinations, 28 days apart, at three different doses. Antibody responses were higher with a higher dose and further increased after the second vaccination, leading to serum-neutralizing activity in all participants. Values were similar to those in the upper half of the distribution of a panel of control convalescent serum specimens. Solicited adverse events that occurred in > 50% included fatigue, chills, headache, myalgia, and pain at the injection site.
Arnold C. How computational immunology changed the face of COVID-19 vaccine development. Nat Med. 2020 Jul 15. PubMed: https://pubmed.gov/32669667. Full-text: https://doi.org/10.1038/d41591-020-00027-9
After more than two decades of work, computational immunology now enables the development of a candidate vaccine in just a few hours. However, no in silico analysis, no matter how high-quality the input and how exacting the computational algorithms, will ever be a substitute for experimental data.
Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020 Jul 15. PubMed: https://pubmed.gov/32669297. Full-text: https://doi.org/10.1126/science.abc8511
Patients differ: Analysing 125 COVID-19 patients, the authors identified three “immunotypes” associated with poorer clinical trajectories versus improving health. A subgroup of patients had T cell activation characteristic of acute viral infection and plasmablast responses reaching > 30% of circulating B cells. However, another subgroup had lymphocyte activation comparable to uninfected subjects. Stable versus dynamic immunological signatures were identified and linked to trajectories of disease severity change. This study provides a compendium of immune response data and also an integrated framework as a “map” for connecting immune features to disease. By localizing patients on an immune topology map built on this dataset, we can begin to infer which types of therapeutic interventions may be most useful in specific patients.
Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020 Jul 15. PubMed: https://pubmed.gov/32668444. Full-text: https://doi.org/10.1038/s41586-020-2550-z
Is there a natural immunity? In this study, T cell responses to structural (nucleocapsid protein, NP) and non-structural (NSP-7 and NSP13 of ORF1) regions of SARS-CoV-2 were analyzed in 36 COVID-19 convalescents. In all of them, CD4 and CD8 T cells recognizing multiple regions of the NP protein were found. Surprisingly, the authors also frequently detected SARS-CoV-2 specific T cells in 37 individuals with no history of SARS, COVID-19 or contact with SARS/COVID-19 patients. These T cells exhibited a different pattern of immunodominance, frequently targeting the ORF-1-coded proteins NSP7 and 13 as well as the NP structural protein. Epitope characterization of NSP7-specific T cells showed recognition of protein fragments with low homology to “common cold” human coronaviruses but conserved amongst animal betacoronaviruses. Thus, infection with betacoronaviruses induces multispecific and long-lasting T cell immunity to the structural protein NP.
2 July
Deming ME, Michael NL, Robb M, et al. Accelerating Development of SARS-CoV-2 Vaccines — The Role for Controlled Human Infection Models. NEJM July 1, 2020. Full-text: https://doi.org/10.1056/NEJMp2020076. Full-text: https://www.nejm.org/doi/full/10.1056/NEJMp2020076
The authors review practical considerations relevant to the development of a SARS-CoV-2 controlled human infection models (CHIMs) and the prerequisites for using such a model. Large, randomized, controlled trials of SARS-CoV-2 vaccines are still the most efficient, generalizable, and scientifically robust path to establishing vaccine efficacy. However, SARS-CoV-2 CHIM development might be able to accelerate the development of later rounds of vaccine candidates.
1 July
Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS and SARS. Cell June 28, 2020. Full-text: https://doi.org/10.1016/j.cell.2020.06.035
The CoV spike receptor-binding domain (RBD) is an attractive vaccine target but is undermined by limited immunogenicity. The authors identified a dimeric form of MERS-CoV RBD that overcomes this limitation and significantly increased the immunogenicity. The RBD-dimer significantly increased neutralizing antibody (NAb) titers compared to conventional monomeric form and protected mice against MERS-CoV infection. This can be a generalizable strategy for beta-CoV vaccine design.
20 June
Fuller DH, Berglund P. Amplifying RNA Vaccine Development. NEJM, June 18, 2020. N Engl J Med 2020; 382:2469-2471. Full-text: https://doi.org/10.1056/NEJMcibr2009737
Recent interest in messenger RNA (mRNA) vaccines has been fueled by methods that increase mRNA stability and protein production and improve delivery. The mRNA vaccines do not need to enter the nucleus to express the antigen. Avoidance of the risk of integration into the host genome is thus considered a comparative advantage. The authors describe new techniques in this field. The most promising seems to be a strategy that is based on two RNA vectors — one retaining the replicase-encoding gene and the other encoding the antigen.
Huo J, Zhao Y, Ren J, et al. Neutralisation of SARS-CoV-2 by destruction of the prefusion Spike. Cell Host Microbe June 19, 2020. Full-text: https://doi.org/10.1016/j.chom.2020.06.010
The monoclonal antibody CR3022 tightly binds the receptor binding domain (RBD) and neutralizes SARS-CoV-2. The highly conserved, structure-stabilising, CR3022 epitope is inaccessible in the prefusion Spike, suggesting that CR3022 binding facilitates conversion to the fusion-incompetent post-fusion state. The mechanism of neutralisation is new and was not seen before for coronaviruses, suggesting that the CR3022 epitope should be a major target for therapeutic antibodies.
24 May
Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 Jun 13;395(10240):1845-1854. PubMed: https://pubmed.gov/32450106. Full-text: https://doi.org/10.1016/S0140-6736(20)31208-3 l (Important)
Open-label Phase I trial of an Ad5 vectored COVID-19 vaccine, using the full-length spike glycoprotein. A total of 108 healthy adults aged between 18 and 60 years from Wuhan, China, were given three different doses. ELISA antibodies and neutralising antibodies increased significantly and peaked 28 days post-vaccination. Specific T cell response peaked at day 14 post-vaccination. Follow-up is still short and the authors are going to follow up the vaccine recipients for at least 6 months, so more data will be obtained. Of note, adverse events were relatively frequent, encompassing pain at injection sites (54%), fever (46%), fatigue (44%) and headache (39%). Phase II studies are underway.
22 April
Callaway E. Hundreds of people volunteer to be infected with coronavirus. Nature 22 April 2020. Full-text: https://www.nature.com/articles/d41586-020-01179-x
What about a ‘human challenge’ vaccine study? Such a trial would be much faster: a much smaller group of young, healthy volunteers would receive a candidate vaccine and then be intentionally infected with the virus, to judge the efficacy of the immunization. No trial is yet planned, but the debate is on. The approach is also gaining some political support.
11 April
Le TT, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nature reviews drug discovery. 09 April 2020. Full-text: https://www.nature.com/articles/d41573-020-00073-5
Brief data-driven overview by seven experts. The conclusion is that efforts are unprecedented in terms of scale and speed and that there is an indication that a vaccine could be available by early 2021. As of 8 April 2020, the global vaccine landscape includes 115 candidates, of which the 5 most advanced candidates have already moved into clinical development, including mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio, LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute. The race is on!
31 March
Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. NEJM March 30, 2020. Full-text: https://doi.org/10.1056/NEJMp2005630
Excellent review on vaccine development. Outlook on new platforms for RNA and DNA vaccines that can be made quickly because they require no culture or fermentation, instead using synthetic processes. Hope and despair.
April 2020