
*** The following text is out-of-date.***
For the latest news about COVID-19, please open the COVID Reference homepage.
Copy-editor: R. Camp
Last revision: 23 May 2021
This chapter is partly not up-to-date!
Find our new Vaccine page here.
Approved Vaccines
Efficacy
Efficacy against B.1.351-like variants
Pregnant women
Breakthrough infections
Adverse events
Unusual blood clots with low blood platelets
Pathophysiology
Clinical presentation
Diagnosis
Treatment
Questions
Shifting strategies
Benefit vs harm
The Johnson & Johnson vaccine
Conclusion
Anaphylactic reaction
Special Topics
Post-exposure SARS-CoV-2 vaccination
One vaccine dose after previous SARS-CoV-2 infection
Delayed booster injection
Two shots, two vaccines
Protection of the non-vaccinated
Single Vaccines
The BioNTech/Pfizer vaccine
History and approval
Efficacy against variants
Adverse events
Adolescents
Children 6 months to 11 years old
Pregnant women
Development
Trivia
The Moderna vaccine
History and approval
Efficacy against variants
Adverse events
Adolescents and children
Development
The AstraZeneca vaccine
History and approval
Efficacy against variants
Adverse events
Adolescents and children
Development
Future
The Johnson & Johnson vaccine
History and approval
Efficacy against variants
Adverse events
Pregnant women
Development
Future
Vaccines approved outside the EU and the US
The Gamaleya vaccine
The Sinovac vaccine
The Sinopharm vaccine
The Bharat vaccine
Other vaccines
Coming vaccines
The Novavax vaccine
History
Efficacy against variants
Adverse events
Children
Development
Trivia
Outlook
Israel
United States
France
In check
References
Approved Vaccines
As of 23 May 2021, four COVID-19 vaccines have been approved or authorized for emergency use in the EU or the US (see also Table 1):
- The BioNTech/Pfizer vaccine. Trade name: Comirnaty™ (tozinameran, formerly known as BNT162b2)
- The Moderna vaccine, also known as mRNA-1273
- The AstraZeneca/University of Oxford vaccine. Trade name: Vaxzevria™/Covishield™ (formerly known as ChAdOx1 nCoV-19, AZD1222)
- The Johnson & Johnson (Janssen) vaccine, also known as Ad26.COV2.S
Outside the EU and the US, four other vaccines have been approved:
- BBIBP-CorV, Sinopharm and the Beijing Institute of Biological Products – first approved in China on 30 December 2020
- Covaxin, Bharat Biotech – first approved in India on 3 January 2021
- Sputnik-V, Gamaleya Research Institute – first approved in Russia, 28 December 2020
- Convidecia, CanSinoBIO – first approved in China, 25 February 2021
| Table 1. SARS-CoV-2 vaccines approved in Europe (EMA) and the US (FDA) | ||||
| Manufacturer Vaccine™ |
Efficacy Storage |
Age | Injections | References |
| BioNTech/Pfizer
Comirnaty™ (Tozinameran, formerly BNT162b2) |
95%
–25°C to -15°C for a max. of two weeks |
16+ years | 2 x 3 weeks apart | Polack 2020 Mulligan 2020FDA EUA FDA briefing doc Sponsor briefing docRecommendation for use |
| Moderna
N.N.™ mRNA-1273
|
94%
–20°C (–4°F) |
18+ years | 2 x 4 weeks apart | Polack 2020 Jackson 2020FDA EUA FDA briefing doc Sponsor briefing docRecommendation for use |
| AstraZeneca & Oxford UniversityVaxzevria™ (formerly AZD1222, ChAdOx1 nCoV-19) |
62-90%
2-8°C (36-46°F) |
18+ years
55+ years 65+ years Suspended (see below*) |
2 x up to 12 weeks apart | Voysey 2020 Folegatti 2020MHRA Decision EMA 20210129 EMA Overview |
| Johnson & Johnson (Janssen)N.N.™Ad26.COV2.S |
67%
2-8°C (36-46°F) |
18 years and older | 1 x | FDA 20210226 EMA 20210311 Stephenson 2021 |
After an unusually frequent occurrence of cerebral sinus vein thromboses less than two weeks after injection of the AstraZeneca vaccine (mostly in younger women), several European countries stopped the use of the vaccine (Netherlands, Denmark, Norway) or restricted its use to people > 55 years of age (France, Canada), > 60 (Germany) or > 65 (Sweden, Finland). German authorities are now considering offering a second injection with another vaccine.
In December 2020, a Belgian minister tweeted the price that the EU had agreed to pay for COVID vaccines (The Guardian). The University of Oxford/AstraZeneca vaccine is the cheapest and Moderna is the most expensive:
- BioNTech/Pfizer: €12
- Moderna/NIAID: $18
- University of Oxford/AstraZeneca: €1.78 (£1.61)
- Johnson & Johnson: $8.50 (£6.30)
Initially, AstraZeneca had pledged it would provide doses on a cost basis for at least as long as the pandemic lasts and in poorer countries in perpetuity. However, according to a newspaper article, an agreement between AstraZeneca and a Brazilian manufacturer seem to define the “Pandemic Period” as ending on July 1, 2021. The period could be extended but only if “AstraZeneca acting in good faith considers that the SARS-COV-2 pandemic is not over” (Financial Times, 8 October 2020).
Efficacy
The currently licensed COVID-19 vaccines offer very good protection against infection with the Wuhan strain and the B.1.1.7 variant. The estimated effectiveness of the BioNTech/Pfizer vaccine after the second dose was 92% for documented infection, 94% for symptomatic COVID-19, 87% for hospitalization, and 92% for severe COVID-19 (Dagan 2021). A protective effect of up to 80% has been shown as soon as two weeks after the first injection (Dagan 2021, Pilishvili 2021) (Table 2).
| Table 2. Effectiveness of the BioNTech/Pfizer vaccine in Israel (2 x 596,618 persons) (Dagan 2021). Find more sub-population data at https://bit.ly/3eMlSfS. | |||
| Vaccine effectiveness | |||
| 14 through 20 days after the first dose | 21 through 28 days after the first dose | 7 days after the second dose and later | |
| Documented infection | 46% | 60% | 92% |
| Symptomatic COVID-19 | 57% | 66% | 94% |
| Hospitalization | 74% | 78% | 87% |
| Severe COVID-19 | 62% | 80% | 92% |
| Death | 72% | 84% | N.N. |
The results of this Phase IV analysis from Israel are important in two ways. First, they describe a COVID-19 vaccine under real-life conditions, matching almost 600,000 vaccinees to an equal number of unvaccinated controls according to demographic and clinical characteristics. This figure is almost 30 times the number of participants in the Phase III study by Polack et al. (n = 21,720; Polack 2020). Second, the trial took place in an epidemiological environment where the B.1.1.7 variant was the dominant lineage. This is comforting news for countries where B.1.1.7 has become or is becoming the dominant strain.
Other Phase IV analyses confirm the efficacy of the COVID-19 vaccines:
- Two weeks after administration of the first COVID-19 vaccine dose, the risk of SARS-CoV-2 infection, hospitalization and death progressively decreases up to about 35 days, both in men and women and in people of different age groups. These are the results of a study by the Italian National Institute of Health (Istituto Superiore di Sanità, ISS) which analyzed 7,370,008 individuals vaccinated as of 4 April 2021. 65% of the study population had received the first injection of the BioNTech/Pfizer vaccine, 6% the first Moderna and 29% the first AstraZeneca injection (Pezzotti 2021) [1]. The authors describe a
- ~80% reduction for the risk of receiving a diagnosis of SARS-CoV-2 infection
- 90% reduction for the risk of hospitalization
- 95% reduction for the risk of death (see Figure 1)
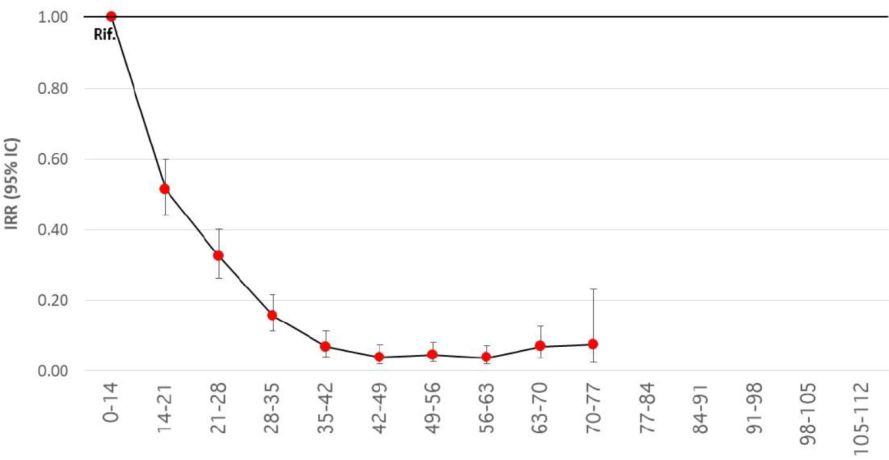
Figure 1. Reduction of the risk of diagnosis and subsequent death at different time intervals from administration of any first dose of the BioNTech/Pfizer, Moderna or AstraZeneca vaccine, starting from the beginning of the vaccination cycle compared to the period 0-14 days from the first dose (reference period).
- Vasileiou 2021, Hall 2021, Public Health England 20210222: The vaccines used in Scotland and England – BioNTech/Pfizer and AstraZeneca – protected well over 80% of vaccinees against COVID-19-related hospitalization at 28-34 days post-vaccination, even aged ≥ 80 years, and even after a single dose.
- Thompson 2021: In a prospective cohort of 3950 health care personnel, first responders, and other essential and frontline workers who completed weekly SARS-CoV-2 testing for 13 consecutive weeks, mRNA (BioNTech/Pfizer or Moderna) vaccine effectiveness of full immunization (≥ 14 days after second dose) was 90% against SARS-CoV-2 infections regardless of symptom status; vaccine effectiveness of partial immunization (≥ 14 days after first dose but before second dose) was 80%.
- In a prospective, UK population-representative cohort study of 373,402 participants aged ≥ 16 years, the odds of new SARS-CoV-2 infection were reduced 65% in the ≥ 21 days since first vaccination with the BioNTech/Pfizer or Oxford-AstraZeneca vaccine (Pritchard 2021). Older and more vulnerable people were as protected as younger healthy individuals. A second dose of the BioNTech/Pfizer vaccine boosted protection further, reducing symptomatic infections by 90% and asymptomatic infections by 70%. Vaccination also reduced SARS-CoV-2 infections with evidence of high viral shedding Ct < 30 (88% reduction after two doses) and with self-reported symptoms (90% reduction after two doses).
The onset of protection for the BioNTech/Pfizer and the Moderna vaccines (both mRNA vaccines) was observed as early as 12 days after the first dose. An analysis of the serological and T cell response after the first dose of the BioNTech/Pfizer vaccine showed that 80% of vaccinees developed spike-binding antibodies at day 10 after the first dose and 100% developed spike-specific T cells at the same time point. The authors suggest that early T cell and binding antibody responses, rather than either receptor blocking or virus neutralizing activity, might be correlates of early protection against COVID-19 (Kalimuddin 2021).
Efficacy against B.1.351-like variants
B.1.351-like variants currently include B.1.351 (first detected in South Africa) and P.1 (Brazil). Both strains harbor the E484K mutation (Tegally 2021, Voloch 2020) which is the “bad boy on the block”. Results from clinical vaccine trials (Table 3) have shown that the level of protection against moderate to severe COVID-19 infection was lower in South Africa where B.1.351 has been the predominant variant of late:
- The Johnson & Johnson vaccine provided a level of protection against moderate to severe COVID-19 infection of 57% in South Africa and 72% in the United States (JNJ 20210129).
- The not yet approved Novavax vaccine candidate provided a level of protection against mild and moderate-to-severe COVID-19 infection of only 49% in South Africa (Novavax 20210311).
- The AstraZeneca vaccine performed poorly in South Africa – no protection against mild-moderate COVID-19 due to B.1.351 (Madhi 2021).
| Table 3. Vaccine efficacy against new variants | ||
| Vaccine manufacturer | Participants | Main efficacy findings |
| Efficacy against B.1.1.7 | ||
| Novavax | 15,203 | 86% efficacy (vs 96% for historical variant) |
| AstraZeneca | 4236 | 75% efficacy (vs 85% for historical variant) |
| Efficacy against B.1.351 | ||
| Johnson & Johnson
(Janssen) |
~10,900 | 57% efficacy (72% in US) |
| Novavax | 4422 | 49% efficacy
– HIV negative: 55% – HIV positive: probably substantially lower |
| AstraZeneca | ~2000 | “minimal protection vs mild-moderate infection” |
These results were anticipated by in vitro studies which showed that B.1.351-like variants have a higher potential for evasion of natural or vaccine-induced immunity than B.1.1.7. A map of all amino acid mutations to the SARS-CoV-2 spike receptor-binding domain (RBD) showed that the site where mutations tended to have the largest effect on antibody-binding and neutralization was E484 (Greaney 2021b). Another study by David H. Ho and colleagues found that the serum of 12 people vaccinated with Moderna’s vaccine and 10 people vaccinated with the BioNTech/Pfizer vaccine was 10 to 12 times less potent against B.1.351 (Wang P 2021). In serum from 20 people previously infected with SARS-CoV-2 the drop in plasma neutralization against B.1.351 was 9-fold. E484K accounted for much of the effect.
P.1, the variant first detected in Brazil, was also more resistant to neutralization by (first-wave) convalescent plasma (de Souza 2021, Wang P 2021, Faria 2021). Plasma from individuals vaccinated with the Chinese CoronaVac vaccine, too, failed to efficiently neutralize P.1 lineage isolates (de Souza 2021).
A recent paper reports that the BioNTech/Pfizer vaccine did not prevent an outbreak of the B.1.351 variant (first detected in South Africa) in a French nursing home; however, it reduced transmission: all unvaccinated residents (5/5), but only half of the vaccinated residents (13/26) were infected (Bailly 2021). The SARS-CoV-2 viral load was significantly higher in non-vaccinated residents (mean cycle threshold (Ct) value: 15, range 12-17) than in vaccinated residents (mean Ct: 21, range: 13-32). The vaccine also reduced disease severity. Among the vaccinated residents who were infected, 2 (15.4%) were asymptomatic and 9 (69.2%) had mild to moderate disease; two individuals (15.4%) had severe disease and died. Among the 5 non-vaccinated residents, 4 progressed to severe disease; one of them died.
The current – preliminary – state-of-knowledge can be summarized as follows:
- While natural and vaccine-induced immunity is likely to protect against infection with B.1.1.7, it may be insufficient to fully protect against B.1.351, P.1, and P.1.617.2.
- However, even in the absence of antibody neutralization, we should expect some T cell protection (Tarke 2021)
- Several vaccines may provide satisfying immunity against SARS-CoV-2 variants
- Most vaccines will probably provide protection against hospitalizations/deaths from these variants
- A booster vaccine against these variants is likely to be effective
Efficacy in people ≥ 65 years
mRNA vaccines have recently been shown to be exquisitely effective also in adults aged ≥ 65 years (Tenforde 2021) with a:
- 94% protection among individuals who were fully vaccinated
- 64% among individuals who were partially vaccinated (defined as onset of COVID-like illness 14 days or later after the first dose but less than 14 days after the second dose)
- No protection during the first 14 days after the first vaccine dose
Half of the patients in this study were 75 years or older.
Pregnant women
An analysis of more than 35,000 pregnant women 16 to 54 years of age showed that injection-site pain was reported more frequently among pregnant women, whereas headache, myalgia, chills, and fever were reported less frequently (Shimabukuro 2021b). Among almost 4000 women enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy. The frequency of miscarriage (Quenby 2021), preterm birth, small size for gestational age, congenital anomalies, and neonatal death didn’t appear to be different from data published before the COVID-19 pandemic.
In a small cohort study, 30 pregnant and 16 lactating women developed both humoral and cellular immune responses after vaccination with the BioNTech/Pfizer or the Moderna vaccine. Vaccine-elicited antibodies were also found in infant cord blood and breast milk (Collier 2021).
Breakthrough infections
Breakthrough infections even among fully vaccinated persons (Hacisuleyman 2021) will be daily bread and butter over the coming months. The clinical course is expected to be generally milder than in unvaccinated individuals. In one recent study, two thirds of breakthrough infections among persons in skilled nursing facilities (SNF) were asymptomatic (Teran 2021) and no facility-associated secondary transmission was identified. Another study estimated that unvaccinated SNF residents and health care personnel (HCP) had 3.0 and 4.1 times the risk of infection compared to vaccinated residents and HCP. Vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective among HCP (Cavanaugh 2021).
Adverse events
Although local or systemic side effects are frequent – mostly pain at injection site, fatigue, headache, muscle pain, joint pain, and sometimes fever during the first 24 to 48 hours after vaccination (Folegatti 2020, Voysey 2020, Jackson 2020, Mulligan 2020, Polack 2020, Baden 2020) – more severe side effects have been in the single-digit range. As a general rule, side effects appear to be more common after the second dose, and younger adults experience more side effects than older adults. The frequency of reported reactions has since been confirmed by real-world observations of more than 3 million people (Chapin-Bardales 2021) through v-safe, a surveillance system for collecting near–real-time data from COVID-19 vaccine recipients in the US.
In the Phase III studies of the BioNTech/Pfizer and Moderna vaccines, serious[2] side effects were equally rare in people who received the vaccine and those who received placebo (Polack 2020, Baden 2020). Anaphylactic reactions may occur in 1 of 100,000 vaccine recipients (see page 22). In the initial trials, no other safety warnings had been found, and the risk of serious adverse effects remains remarkably low after administration of a billion vaccine doses by the end of April 2021. In mid-February, just 20 cases of patients with thrombocytopenia and bleeding without thrombosis after vaccination with the mRNA–based vaccines produced by BioNTech/Pfizer and Moderna had been reported (Lee EJ 2021).
Then, still in February, suddenly, the first and until now only truly worrisome adverse event of COVID-19 vaccines was reported: life-threatening thromboses, together with thrombocytopenia and sometimes bleeding that occurred as early as 4 days after injection of the AstraZeneca vaccine.
Unusual blood clots with low blood platelets
As of 26 April, several hundred cases of unusual thrombosis in veins in the brain (cerebral sinus vein thromboses, CSVT[3]), the abdomen (splanchnic vein thrombosis) and in arteries were reported after the first injection of the AstraZeneca vaccine. The first symptoms appeared as early as five days and as late as a month after vaccination. Cases of the new syndrome – vaccine-induced immune thrombotic thrombocytopenia (VITT) or thrombosis-thrombocytopenia syndrome (TTS) – have been reported from several countries, including Germany and Austria (Greinacher 2021), Norway (Schultz 2021), France (ANSM 20210416), and the UK (MHRA 20210401, Scully 2021). By April 21, the Paul-Ehrlich-Institut (PEI), Germany’s vaccine regulator, had registered 59 cases (14 men and 45 women) of this syndrome. Of the 43 women for whom the time interval between vaccination and the onset of symptoms is known, 38 were between 22 and 59 years old. Twelve of the 14 men affected were 20 to 59 years old, the other two were between 60 and 70. The symptoms began in 57 of the 59 cases within 29 days of the vaccination. Twelve people died, six men and six women. With around 4.2 million vaccinated with the AstraZeneca vaccine, the risk for vaccine-induced immune thrombotic thrombocytopenia (VITT) was around one case in 70,000 vaccinated; for women, the risk was higher. In Norway, five health care workers 32 to 54 years of age had venous thrombosis and thrombocytopenia 7 to 10 days after receiving the first dose of the AstraZeneca vaccine. Three patients died. The five cases occurred in a population of around 130,000 vaccinated persons (1:26,000) (Schultz 2021; see also Pottegård 2021, Hunter 2021).
Up to 14 April 2021, UK authorities were aware of 168 cases of major thromboembolic events with concurrent thrombocytopenia following vaccination with the AstraZeneca vaccine. These events occurred in 93 women (55%) and 75 men aged from 18 to 93 years. A total of 32 deaths occurred (fatality rate: 19%) (MHRA 20210422). Cerebral venous sinus thrombosis was reported in 77 cases (average age 47 years) and 91 had other major thromboembolic events (average age 55 years) with concurrent thrombocytopenia. With 21.2 million administered by 14 April, the risk was 1 in 126,000 administrations. The data also suggest that there was a higher incidence in younger adult age groups. The MHRA advised that this “evolving evidence should be taken into account when considering the use of the vaccine”.
Young age and female gender were initially thought to be at increased risk for VITT; in the study from Germany and Austria, 9 of the 11 patients were women and most were relatively young adults (median age: 36; range, 22 to 49). However, higher age and male gender should not induce physicians to exclude VITT. French reports describe a total of 34 cases of atypical thrombosis cases out of more than four million injections, including 11 deaths (ANSM 20210517, 17 May). The mean age of recent cases was in the 60s and half of them were men (ANSM 20210416, 16 April; ANSM 20210423, 23 April).
Pathophysiology
A tentative mechanism by which the AstraZeneca vaccine might trigger an immune response leading to VITT (–> highly reactive anti-PF4 antibodies with downstream FcγIIa receptor-dependent amplification; –> recruitment of neutrophils; –> neutrophil activation and NETs formation; –> triggering a prothrombotic response) has recently been proposed in a pre-print (Greinacher 2021b).
Clinical presentation
The clinical picture of thrombocytopenia and thrombotic complications at unusual sites one to four weeks after the administration of the AstraZeneca vaccine reflects an immunologic pattern similar to that of severe heparin-induced thrombocytopenia (HIT[4]), a prothrombotic disorder caused by platelet-activating antibodies that recognize multi-molecular complexes between cationic PF4 and anionic heparin (Greinacher 2015). The clinical presentation of vaccine-induced immune thrombotic thrombocytopenia (VITT) may be entirely unspecific (headache, backache, chills, fever, nausea, epigastric discomfort) or highly suggestive (stroke or reduced consciousness after three days of headache; Schultz 2021), especially when physicians are informed about administration of the AstraZeneca vaccine in the previous 4 weeks. A paper from Germany and Austria describes thrombotic events including cerebral venous thrombosis (in 9 patients), splanchnic vein thrombosis (in 3 patients), pulmonary embolism (in 3 patients), and other types of thrombi (in 4 patients); 5 of 10 patients had more than one thrombotic event (Greinacher 2021). All patients presented with concomitant thrombocytopenia (median nadir of platelet count, approximately 20,000 per cubic millimeter; range, 9000 to 107,000). A paper from Norway describes five cases that occurred 7 to 10 days after the first injection of the AstraZeneca vaccine. Four of the patients had severe cerebral venous thrombosis with intracranial hemorrhage (Schultz 2021). Three patients died.
Diagnosis
In the context of mass vaccination with the AstraZeneca vaccine, clinicians should be aware that rarely, venous or arterial thrombosis can develop at unusual sites within the first months after vaccination. Clinicians should have a low threshold for requesting ELISA testing for PF4–polyanion antibodies, including confirmatory functional testing, in patients who have
- Single or multiple thromboses in unusual locations:
- Cerebral venous sinus thrombosis (CVST)
- Thrombosis of portal, splanchnic, or hepatic veins
- Pulmonary emboli
- Acute arterial thromboses
- Low platelet counts. In the Greinacher study, the mean was 35,000 per mm3 (range, 8000 to 107,000; Greinacher 2021)
- High levels of d-dimers
- Low levels of fibrinogen
A suspicion of VITT is confirmed by the presence of anti-PF4 antibodies (Juhl 2006, Selleng 2015) with an approved PF4 ELISA (see also Oldenburg 2021).
To detect PF4-specific antibodies in patients with suspected VITT, the use of a sensitive, quantitative, immunologic test is strongly recommended. Rapid immunoassays should be avoided (Vayne 2021).
Positive PF4/polyanion enzyme immunoassays (EIAs) can occur after SARS-CoV-2 vaccination with both mRNA- and adenoviral vector-based vaccines. In a recent study, the EIA was found to be positive in 19 of 281 vaccinees (all: 6.8%; BioNTech/Pfizer: 5.6%; AstraZeneca: 8.0%); however, optical densities were mostly between 0.5-1.0 units (reference range, < 0.50) and none of the PF4/polyanion EIA-positive samples induced platelet activation in the presence of PF4 (Thiele 2021). In most cases, these antibodies are likely to have only minor (if any) clinical relevance.
Find a diagnostic algorithm and therapeutic strategies for the management of suspected VITT at Greinacher 2021.
As for now, no predisposing factors for VITT have been identified. There is no indication that a history of thrombosis, HIT or other risk factors (i.e., birth control pills) increase the risk of VITT.
Treatment
VITT is treatable if identified quickly. On 29 March, the German GTH (Gesellschaft für Thrombose- und Hämostaseforschung – Society for Thrombosis and Hemostasis Research) suggested that the prothrombotic pathomechanism could likely be interrupted by the administration of high-dose intravenous immunoglobulins (IVIG), i.e., at a dose of 1 g per kg of body weight daily on two consecutive days (Oldenburg 2021). Intravenous immunoglobulin and high-dose glucocorticoids can improve the platelet count within days.
It is yet unclear whether delaying anticoagulation until after initial disease control with IVIG or plasma exchange is beneficial (Scully 2021). Reluctance to start anti-coagulation with non-heparin anti-coagulant agents such as argatroban, danaparoid, or fondaparinux may be tempered by administering high dose of IVIG to raise the platelet count, especially when a patient presents with severe thrombocytopenia and thrombosis, such as cerebral venous thrombosis (Greinacher 2021).
Treatment with platelet transfusions should be avoided because they would provide a substrate for further antibody-mediated platelet activation and coagulopathy (Scully 2021).
With earlier recognition and aggressive treatment, the high mortality rate of VITT is likely to decrease.
Questions
Over the coming weeks and months, we might see more unusual clinical pictures in previously healthy individuals after the administration of the AstraZeneca or the Johnson & Johnson vaccine, such as, for example, superior ophthalmic vein thrombosis (SOVT) + immune thrombocytopenia + ischaemic stroke (Bayas 2021). In many cases, it will be delicate to establish or refute a causal relationship with the vaccination.
The following questions, recently summarized by Douglas Cines and James Bussel (Cines & Bussel 2021), will need to be addressed soon:
- What component or components of the vaccine (adenoviral sequence, spike protein, or other component) elicit this new (or recall) response to a seemingly unrelated host protein, PF4?
- What is the risk after re-vaccination?
- How do VITT antibodies compare with the anti-PF4–related antibodies that are present after SARS-CoV-2 infection, which have been described in patients who were suspected to have heparin-induced thrombocytopenia?
- Is PF4 a bystander component within an immune complex that activates platelets, or does it contribute directly to clot propagation?
- Does the atypical distribution of thrombi relate to antigen localization or vascular response?
- Is thrombosis propagated along vascular and hematopoietic surfaces that release diverse anionic co-factors, as in heparin-induced thrombocytopenia?
And yet another question:
- Do mild – undiagnosed – forms of VITT exist? If yes, could these predispose people to clinically relevant thrombotic events in the future?
Shifting strategies
VITT has devastating effects for otherwise healthy young adults and requires a thorough risk–benefit analysis (Schultz 2021). In late March, several European countries stopped using the AstraZeneca vaccine (Denmark, Norway) or restricted its use to people > 55 years of age (France, Canada), > 60 (Germany) or > 65 (Sweden, Finland). In Spain, where rules change frequently, it is restricted to those between 60 and 69.
On 7 April, EMA announced that unusual thrombosis and thrombocytopenia should be listed as very rare side effects of the AstraZeneca vaccine (EMA 20210407). Healthcare professionals should tell people receiving the vaccine that they must seek medical attention if they develop:
- symptoms of blood clots such as shortness of breath, chest pain, leg swelling, persistent abdominal pain
- neurological symptoms such as severe and persistent headaches and blurred vision
- petechiae beyond the site of vaccination after a few days.
Although the EMA stated that the overall benefits of the AstraZeneca vaccine in preventing COVID-19 outweighed the risks of side effects, the agency also specified that the “use of the vaccine during vaccination campaigns at national level will also take into account the pandemic situation and vaccine availability in the individual Member State (EMA 20210407).” The British Joint Committee on Vaccination and Immunisation (JCVI) issued a less ornate and more cautious recommendation, advising that it is preferable for adults aged less than 40 years to be offered an alternative COVID-19 vaccine, if available (JCVI 20210507) (unless they have underlying health conditions and only if this does not cause substantial delays in being vaccinated). Some physicians, especially those in private practice, might feel more comfortable administering alternative vaccines even in those older than 40 years.
Benefit vs harm
EMA’s human medicines committee analyzed the vaccine’s benefits and the risk of unusual blood clots with low platelets in different age groups in the context of the monthly infection rates: low (55 per 100,000 people = 18 daily infections per 1,000,000 people), medium (401 per 100,000 people = 133 daily infections per 1,000,000 people) and high (886 per 100,000 people = 295 daily infections per 1,000,000 people) (EMA 20210423). The following three tables show the potential benefits and harms of the AstraZeneca for a low (Table 4a), medium (Table 4b) and high (Table 4c) transmission rate scenario.
| Table 4a. Weighing up the potential benefits and harms of the AstraZeneca vaccine in a low transmission rate scenario*. Expected VITT cases and number of prevented 1) hospitalizations, 2) ICU admissions and 3) deaths after vaccination with the AstraZeneca vaccine. | |||||
| Prevented (considering an 80% vaccine effectiveness over a period of four months) |
|||||
| Age group | Thromboses (VITT**; also called TTP) | Hospitalizations | ICU admission | Deaths | |
| 20–29 | 1.9 | 4 | 0 | 0 | |
| 30–39 | 1.8 | 5 | 0 | 0 | |
| 40–49 | 2.1 | 6 | 1 | 1 | |
| 50–59 | 1.1 | 10 | 1 | 1 | |
| 60–69 | 1 | 19 | 3 | 3 | |
| 70–79 | 0.5 | 45 | 6 | 14 | |
| 80+ | 0.4 | 151 | 13 | 90 | |
* Low infection rate defined a monthly incidence of 55/100,000 population which corresponds to a daily incidence of 18/1,000,000. Examples: US: 23 March 2020; France: 4 August 2020; Germany: 17 September 2020; Italy: 27 August 2020; Spain: 16 July 2020. From: AstraZeneca’s COVID-19 vaccine: benefits and risks in context. Medicines Agency (EMA) 2021, published 23 April (EMA 20210423)
** VITT: Vaccine-induced thrombosis with thrombocytopenia
= TTP: Thrombosis with thrombocytopenia
| Table 4b. Weighing up the potential benefits and harms of the AstraZeneca vaccine in a medium transmission rate scenario*. Expected VITT cases and number of prevented 1) hospitalizations, 2) ICU admissions and 3) deaths after vaccination with the AstraZeneca vaccine. | |||||
| Prevented (considering an 80% vaccine effectiveness over a period of four months) |
|||||
| Age group | Thromboses (VITT**; also called TTP) | Hospitalizations | ICU admission | Deaths | |
| 20–29 | 1.9 | 37 | 3 | 0 | |
| 30–39 | 1.8 | 54 | 5 | 2 | |
| 40–49 | 2.1 | 81 | 10 | 7 | |
| 50–59 | 1.1 | 114 | 15 | 8 | |
| 60–69 | 1 | 183 | 28 | 25 | |
| 70–79 | 0.5 | 278 | 39 | 87 | |
| 80+ | 0.4 | 332 | 29 | 197 | |
* Medium infection rate defined a monthly incidence of 401/100,000 population which corresponds to a daily incidence of 133/1,000,000. Examples: US: 22 Septemer 2020; France: 18 September 2020; Germany: 27 October 2020; Italy: 18 October 2020; Spain: 27 March 2021. From: AstraZeneca’s COVID-19 vaccine: benefits and risks in context. Medicines Agency (EMA) 2021, published 23 April (EMA 20210423)
** VITT: Vaccine-induced thrombosis with thrombocytopenia
= TTP: Thrombosis with thrombocytopenia
| Table 4c. Weighing up the potential benefits and harms of the AstraZeneca vaccine in a high transmission rate scenario*. Expected VITT cases and number of prevented 1) hospitalizations, 2) ICU admissions and 3) deaths after vaccination with the AstraZeneca vaccine. | |||||
| Prevented (considering an 80% vaccine effectiveness over a period of four months) |
|||||
| Age group | Thromboses (VITT**; also called TTP) | Hospitalizations | ICU admission | Deaths | |
| 20–29 | 1.9 | 64 | 6 | 0 | |
| 30–39 | 1.8 | 81 | 8 | 3 | |
| 40–49 | 2.1 | 122 | 15 | 10 | |
| 50–59 | 1.1 | 208 | 28 | 14 | |
| 60–69 | 1 | 324 | 50 | 45 | |
| 70–79 | 0.5 | 547 | 78 | 172 | |
| 80+ | 0.4 | 1239 | 110 | 733 | |
* High infection rate defined a monthly incidence of 886/100,000 population which corresponds to a daily incidence of 295/1,000,000. Examples: US: 3 November 2020; France: February 2020; Germany: Christmas 2020; Italy: 2 March 2021; Spain: 21 October 2020. From: AstraZeneca’s COVID-19 vaccine: benefits and risks in context. Medicines Agency (EMA) 2021, published 23 April (EMA 20210423)
** VITT: Vaccine-induced thrombosis with thrombocytopenia
= TTP: Thrombosis with thrombocytopenia
These figures show how the risks outweigh the benefits of the vaccine 1) the lower the infection rates and 2) the younger the recipients. In other words:
- For younger people, the risk-benefit balance is worse than for older people
- For people living in an environment with low infection rates the risk-benefit balance is worse than for people in an environment with high-infections rates
It is evident that as more young people become eligible to be vaccinated, alternative vaccines (i.e., BioNTech/Pfizer, Moderna) will become more attractive.
The Johnson & Johnson vaccine
Cases of cerebral venous sinus thrombosis (CVST) concomitant with thrombocytopenia have also been described after vaccination with the Johnson & Johnson vaccine (Muir 2021, Sadoff 2021). After a short pause (FDA 20210413), the FDA and the CDC recommended on 23 April to resume the use of the Johnson & Johnson vaccine (FDA 20210423). At that time, the agencies were aware of 15 cases reported to the Vaccine Adverse Event Reporting System VAERS. All cases occurred in women between the ages of 18 and 59, with a median age of 37 years. Symptom onset was between 6 and 15 days after vaccination.
Conclusion
After a “plausible” link (EMA 20210407) between the AstraZeneca vaccine and rare life-threatening thromboses together with thrombocytopenia, it is unclear if the vaccine will be approved by the FDA. If it is approved, it is unclear if it will be used in the US – the country has a huge supply of alternative vaccines. On 26 April, a senior US administration official was quoted saying that there could be “up to 60 million doses of the AstraZeneca vaccine available to be shared with other countries in the next two months” (Collins 2021).
A relatively low number of cerebral sinus vein thromboses and splanchnic vein thromboses have reshaped the landscape of COVID vaccines. In the European Union, some countries have stopped using the AstraZeneca vaccine (Denmark) or will lend all of its more than 200,000 doses of AstraZeneca to neighbouring Iceland and Sweden (Norway). Other countries restrict the use of the vaccine to people over 55, 60 or 65. The European Union has not canceled its existing orders of the AstraZeneca and Johnson & Johnson vaccines, but signaled it might not be going to be placing more (NYTimes 20210414). As COVID vaccine scarcity will soon tip over into vaccine abundance in a growing number of countries, the future market for the AstraZeneca vaccine will need to be defined.
Anaphylactic reaction
On December 8, 2020, within 24 hours of the start of the UK vaccination program, probable cases of anaphylaxis were reported in two women in their forties, who had known food and drug allergies and were carrying auto-injectable epinephrine (Castells 2020). One week later, a 32-year-old female health care worker in Alaska who had no known allergies presented with an anaphylactic reaction within 10 minutes of receiving the first dose of the vaccine. Since then, several more cases of anaphylaxis associated with the Pfizer mRNA vaccine have been reported after vaccination of almost 2 million health care workers, and the incidence of anaphylaxis associated with the Pfizer SARS-CoV-2 mRNA vaccine appears to be approximately 10 times as high as the incidence reported with all previous vaccines, at approximately 1 in 100,000, as compared to 1 in 1,000,000 (Castells 2020, Shimabukuro 2021).
An analysis of the constituents of mRNA vaccines shows that an anaphylactic reaction may be due to several factors which cannot be determined in clinical practice (see Risma 2021). A recent study of three individuals with a history of PEG allergy and three healthy controls found that the BioNTech/Pfizer vaccine induced positive skin tests in PEG allergic patients, whereas traditional PEG skin testing was negative in two of three patients. As an effect could be induced by PEGylated liposomal doxorubicin, the authors suggest that PEGlyated lipids within nanoparticles, and not PEG in its native state, could be a potential trigger of anaphylaxis to the BioNTech/Pfizer vaccine (Troelnikov 2021).
However, it may still be possible to safely vaccinate people with allergies to vaccine components after assessing patients who report allergy to a vaccine, injectable medication, or PEG. Consult an allergist who might triage patients into those able to go ahead with vaccination with the routine 15 minutes of observation, those requiring 30 minutes of observation, and those who require skin testing to PEG and polysorbate before vaccination (Glover 2021, Mustafa 2021).
The CDC recommends that appropriate medical treatment for severe allergic reactions be immediately available in the event that an acute anaphylactic reaction occurs following administration of an mRNA COVID-19 vaccine (CDC 20201231, CDC 20210303). In particular, persons without contraindications to vaccination who receive an mRNA COVID-19 vaccine should be observed after vaccination for the following time periods:
- 30 minutes: Persons with a history of an immediate allergic reaction of any severity to a vaccine or injectable therapy and persons with a history of anaphylaxis due to any cause.
- 15 minutes: Everyone else
Special Topics
Post-exposure SARS-CoV-2 vaccination
Could post-exposure vaccination with SARS-CoV-2 vaccines be able to mitigate COVID-19 disease? Claude Muller of the Luxembourg Institute of Health argues that there might be enough time for protective vaccine effects to set in (Muller 2021):
- The time from SARS-CoV-2 infection to hospitalization is around two weeks:
- Incubation time of SARS-CoV-2 infection: 5 days (Elias 2021)
+
-
- Time from symptom onset to hospitalization: around 7 to 10 days
- Partial protection from mRNA vaccines has been shown as early as two weeks after the first vaccine dose (Polack 2020, Dagan 2021)
In particular, individuals with a long incubation period could benefit from post-exposure vaccination. While a large randomized control trial would be needed to demonstrate the efficacy of this approach, post-exposure SARS-CoV-2 vaccination would cause no harm and could only benefit the vaccine recipients (Muller 2021). Post-exposure vaccination is not new – protection is quite high in a number of infectious diseases (hepatitis A, 85%; hepatitis B, 85%; measles, 83%; varicella, 67%; smallpox, 45%; and mumps, 38%) (Gallagher 2019).
One vaccine dose after previous SARS-CoV-2 infection
Current evidence indicates that only one vaccine dose is needed to maximize immune protection in individuals who survived a previous SARS-CoV-2 infection (Manisty 2021, Krammer 2021, Reynolds 2021). In these cases, the pre-vaccination SARS-CoV-2 infection is analogous to immune priming and the first vaccine dose analogous to the (second) booster injection. It would even seem that protection provided by a previous SARS-CoV-2 infection plus a single BioNTech/Pfizer vaccine dose is superior to ‘No previous COVID-19’ plus two vaccine doses. A high degree of protection provided by 1) a pre-vaccination SARS-CoV-2 infection plus 2) one vaccine dose has been suggested/shown by:
- Antibody titers measured in participants of clinical mRNA vaccine trials (Krammer 2021, Saadat 2021)[5]. In the Krammer study, no increase in antibody titers was observed in people with pre-vaccination SARS-CoV-2 infection who received the second vaccine dose.
- T and B cell responses after a single dose of the BioNTech/Pfizer vaccine (Reynolds 2021). A single dose showed:
- Enhanced T cell immunity
- Antibody secreting memory B cell response to spike
- Effective neutralizing antibodies against the B.1.1.7 and B.1.351 variants (by comparison, a single vaccine dose without prior infection showed only reduced immunity against variants)
- A significant increase of all components of the humoral response with serum neutralizing activities against variants of concern comparable to or greater than neutralizing activity achieved by vaccination of naïve individuals against the historical strain (Wang Z 2021)
- A study that measured antibody and memory B cell responses in 33 SARS-CoV-2 naïve and 11 SARS-CoV-2 recovered subjects (Goel 2021)
- A study of 500 employees of a 350-bed hospital in Israel (Abu Jabal 2021)
- A study of 102 residents from nursing homes in Montpellier, France (Blain 2021)
- A study of 124 Italian healthcare professionals (Levi 2021)
- A study of SARS-CoV-2 spike-specific T and B cell responses, as well as specific IgA, IgG, IgM and neutralizing antibody titers in 22 individuals in Florence, Italy, 11 of which had a previous history of SARS-CoV-2 infection (Mazzoni 2021)
- A study of 51 health-care workers in London (Manisty 2021)
A recent preprint reports that T cells from individuals with pre-vaccination SARS-CoV-2 infection differed from those of infection-naive vaccinees (five of the participants had received the BioNTech/Pfizer vaccine and three the Moderna one) (Neidleman 2021). Compared to SARS-CoV-2-naive individuals, previously infected people might even have a superior long-term persistence of nasopharynx-homing SARS-CoV-2-specific T cells.
In SARS-CoV-2 recovered individuals, the second vaccine dose often had little effect on the immune response (Goel 2021, Painter 2021).
Table 5 presents 6 situations: 1) people with or without previous SARS-CoV-2 infection who 2) receive no, one or two vaccine injections.
| Table 5. Vaccination after previous SARS-CoV-2 infection | ||||
| Scenario | Previous SARS-CoV-2 infection? |
First dose of vaccine | Second dose of vaccine | Immunology |
| 1 | No | No | No | No protection |
| 2 | No | Yes | No | Protection starting around two weeks after the first injection. In one study, 92% of vaccinees (n = 475) had detectable anti-SARS-CoV-2 spike IgG three weeks after the first dose of BioNTech/Pfizer (Abu Jabal 2021). |
| 3 | No | Yes | Yes | Excellent protection (Dagan 2021). |
| 4 | Yes | No | No | (Some) protection against future SARS-CoV-2 infection, possibly even against variant strains such as B.1.1.7 and B.1.351 (Reynolds 2021), but protection probably not as good as in Scenario 3. |
| 5 | Yes | Yes | No | Rapid antibody response after one dose of either the BioNTech/Pfizer or the Moderna vaccine. Probably even better protection than in Scenario 3. A previous SARS-CoV-2 infection is analogous to immune priming – and a single vaccine dose acts as booster injection (Krammer 2021, Abu Jabal 2021, Saadat 2021, Manisty 2021, Goel 2021, Reynolds 2021), even in nursing home residents (Blain 2021). |
| 6 | Yes | Yes | Yes | After a previous SARS-CoV-2 infection, a second vaccine injection would seem to offer no additional protection (Goel 2021). Give the second injection to another person. |
In summary, people with prior SARS-CoV-2 infections
- Benefit from vaccination and should always be vaccinated
- Should probably receive only one dose of vaccine
- To make this dose available for another individual
- To avoid adverse events from the second dose. People with pre-existing immunity may experience systemic side effects such as fatigue, headache, chills, muscle pain, fever, and joint pain with considerably higher frequency than people without pre-existing immunity (Krammer 2021).
It will be interesting to monitor immunity to natural infection and following vaccination over time and show whether differences in vaccine immune response between previously SARS-CoV-2 infected or SARS-CoV-2 naïve individuals are maintained over time.
Delayed booster injection
The debate about whether delaying the second booster vaccine dose is a risk – “extended prime-boost interval” strategy chosen by the UK to vaccinate a higher percentage of the population quicker and to maximize the number of people who would be partially protected from hospitalization and death – may be about to be settled. In a study of 175 people who were aged over 80 and living independently and who received the BioNTech/Pfizer vaccine, peak antibody levels were 3.5 times higher in those who received the booster dose 12 weeks after the first dose when compared to those who received it after 3 weeks (Parry 2021 – PR1, PR2). Further studies will have to show whether these findings can be repeated in younger people and whether the enhanced immune responses seen after an extended prime-boost interval will help sustain immunity over the longer term.
Two shots, two vaccines
The Com-COV trial compares the four possible prime-boost combinations of the BioNTech/Pfizer vaccine and the AstraZeneca vaccine. The preliminary reactogenicity data show that among the participants who received the boost vaccine 28 days after the first dose, both heterologous vaccine schedules (BioNTech/Pfizer + AstraZeneca or AstraZeneca + BioNTech/Pfizer) induced greater systemic reactogenicity following the boost dose than homologous schedules (BioNTech/Pfizer + BioNTech/Pfizer or AstraZeneca + AstraZeneca); this was accompanied by more frequent use of paracetamol (see Table 6) (Shaw 2021). Most of this increase in reactogenicity was observed in the 48 h after the second dose. The authors of the study suggest that routine prophylactic use of paracetamol could help mitigate these effects. They also note that the participants in this trial were aged 50 years and older and that reactogenicity could be higher in younger individuals. Data about the primary immunological outcome are expected in June.
| Table 6. Feverishness* and paracetamol use after the booster dose in homologous and heterologous vaccine schedules | |||
| Prime/Boost (n) | Feverishness | Paracetamol use | |
| BioNTech/Pfizer + BioNTech/Pfizer | 118/117 | 21% | 41% |
| AstraZeneca + AstraZeneca | 115/112 | 10% | 36% |
| AstraZeneca + BioNTech/Pfizer | 114/110 | 34% | 57% |
| BioNTech/Pfizer + AstraZeneca | 115/114 | 41% | 60% |
* Defined as a self-reported feeling of feverishness. Similar increases were observed for chills, fatigue, malaise, headache, joint and muscle ache.
The Spanish CombivacS study reported similar results. The study enrolled 673 volunteers who had received a first dose of the AstraZeneca vaccine. After 8 to 12 weeks, 441 individuals received the BioNTech/Pfizer vaccine for their second dose and 232 received a second AstraZeneca injection. In the BioNTech/Pfizer group, the neutralizing antibody titers rose seven-fold, as compared with three-fold in the AstraZeneca group (ISCIII 20210518). Less than 2% of study participants reported severe side effects, mostly headaches, general malaise and muscle pain.
In the future, such a vaccination strategy, also known as ‘heterologous prime and boost’, may simplify vaccination campaigns in countries with fluctuating vaccine supplies.
Protection of the non-vaccinated
Preliminary data suggest that vaccinating 82% of a vulnerable nursing home population – while continuing to use face masks and other infection-control measures! – may be highly protective for the remaining 18% of unvaccinated residents (see Table 7). The study included 22,232 residents of 280 nursing homes across 21 US states, 18,242 (82%) of whom received at least one dose of mRNA vaccine (80.4% BioNTech/Pfizer, 19.6% Moderna) and 13,048 of these (71.5%) also received the second dose (White 2021). Most infections were asymptomatic, both in vaccinated and unvaccinated residents.
| Table 7. Incident SARS-CoV-2 infection among 3990 unvaccinated nursing home residents | ||||
| Total | Asymptomatic SARS-CoV-2 infection | Symptomatic SARS-CoV-2 infection | Percent of infected residents who were asymptomatic | |
| Positive test | ||||
| at 0-14 days* | 173 (4.3%) | 115 (2.9%) | 58 (1.5%) | 66.5 |
| at 15-28 days* | 69 (1.7%) | 42 (1.1%) | 27 (0.7%) | 60.9 |
| at 29-42 days* | 16 (0.4%) | 13 (0.3%) | 3 (0.1%) | 81.2 |
| at > 42 days | 12 (0.3%) | 10 (0.3%) | 2 (0.1%) | 83.3 |
* After first vaccination at the nursing home
Single Vaccines
The BioNTech/Pfizer vaccine
History and approval
In November 2020, the German company BioNTech and the New York-based Pfizer made history by presenting data which indicated that their vaccine tozinameran (formerly BNT162b2; trade name: Comirnaty™) had an extraordinary efficacy of over 90%. Four months later, these results were reproduced in a spectacular real-life analysis of almost 1.2 million people in Israel. The estimated effectiveness of the BioNTech/Pfizer vaccine after the second dose was 92% for documented infection, 94% for symptomatic COVID-19, 87% for hospitalization, and 92% for severe COVID-19 (Table 8) (Dagan 2021). The vaccine has gained full approval or authorization for emergency use (people 16 years of age) in more than 100 countries. In May, Canada and the US authorized the vaccine for children aged 12 to 15 (Health Canada 20210505, Wallace 2021).
| Table 8. Effectiveness of the BioNTech/Pfizer vaccine in Israel (2 x 596,618 persons) (Dagan 2021) | |||
| Vaccine effectiveness | |||
| 14 through 20 days after the first dose | 21 through 28 days after the first dose | 7 days after the second dose and later | |
| Documented infection | 46% | 60% | 92% |
| Symptomatic COVID-19 | 57% | 66% | 94% |
| Hospitalization | 74% | 78% | 87% |
| Severe COVID-19 | 62% | 80% | 92% |
| Death | 72% | 84% | N.N. |
The BioNTech/Pfizer vaccine is a lipid nanoparticle–formulated (Pardi 2015) nucleoside-modified RNA vaccine (Karikó 2008; see also Karikó 2005 + Karikó 2012 + Karikó by Wired; Karikó by The New York Times) that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full length spike protein (Wrapp 2020). A Phase III trial demonstrated that two 30 μg doses given three weeks apart conferred 95% protection against COVID-19 in persons 16 years of age or older (Polack 2020). Of 170 confirmed COVID-19 cases, 162 occurred in the placebo group and 8 in the vaccine group. Efficacy was consistent across age, gender, race and ethnicity. In particular, the observed efficacy in adults over 65 years of age was above 94%. Safety over a median of 2 months was similar to that of other viral vaccines.
Researchers involved in the development of tozinameran had previously published Phase I safety and immunogenicity data (Walsh 2020). Two 30 μg doses had been shown to elicit high SARS-CoV-2 neutralizing antibody titers and robust antigen-specific CD8+ and Th1-type CD4+ T cell responses (Sahin 2020, Mulligan 2020).
Administration of the BioNTech/Pfizer vaccine swiftly started in many countries. On 31 December, WHO listed the Comirnaty COVID-19 mRNA vaccine for emergency use, making the BioNTech/Pfizer vaccine the first to receive emergency validation from WHO (WHO 20201231). Countries that do not have the means to rigorously assess the efficacy and safety of vaccines could now take advantage of the WHO EV and begin rolling out their vaccination programs.
Two press articles narrate the background of the BioNTech/Pfizer vaccine development (LaFraniere 2020) and how BioNTech/Pfizer make their vaccine (Cott 2021).
Unopened thawed BioNTech/Pfizer vials can be stored at 2-8°C (i.e., in a normal fridge after taking out of deepfreeze conditions) for up to 31 days (EMA 20210517).
Efficacy against variants
The BioNTech/Pfizer vaccine is effective against the B.1.1.7 variant – as a matter of fact, B.1.1.7 was the dominant lineage in Israel when the vaccination campaign started that would later provide the data for the Dagan study. The vaccine has also now been shown to be effective against B.1.351 (first detected in South Africa). In Qatar, in a real-world test, the effectiveness against any B.1.351 infection was 75%, approximately 20 percentage points lower than the effectiveness reported in studies from Israel (Dagan 2021, Haas 2021); however, effectiveness against severe, critical, or fatal disease was well over 90% (Abu-Raddad 2021).
Preliminary in vitro data had already suggested that the SARS-CoV-2 vaccines would retain activity against the B.1.351 (first detected in South Africa) and P.1 (Brazil) (Liu Y 2021, Lustig 2021). Individuals with prior infection showed excellent T cell immunity, antibody secreting memory B cells and neutralizing antibodies effective against B.1.1.7 and B.1.351 (Reynolds 2021). Other variants such as B.1.526 (New York), B.1.429 (California), and B.1.1.7+E484K (England) also seem to remain susceptible to neutralizing antibodies elicited by the BioNTech/Pfizer vaccine (Liu Y 2021b).
For the B.1.617 variant first identified in India (Vaidyanathan 2021), in vitro studies showed that B.1.617 evaded antibodies induced by infection (15 ICU COVID-19 patients) or vaccination (15 recipients of the BioNTech/Pfizer vaccine), although to a moderate degree (Hoffmann 2021). In another study, samples from convalescent patients and from individuals vaccinated with the the BioNTech/Pfizer or Moderna vaccines still had neutralizing activity against B.1.617.1 (although the variant was 7 times more resistant to neutralization) (Edara 2021). One B.1617 mutation, P681R, favored syncytium formation, potentially contributing to the increased pathogenesis observed in hamsters and contributing to the rapid spread of B.1.617 (Ferreira 2021).
The variant B.1.617.2 (a sub-lineage of B.1.617) is now considered a variant of concern (Public Health England 20210507, Public Health England 20210513, NYTimes 20210510), on par with B.1.1.7, B.1.351 and P.1. It seems to be at least as transmissible as B.1.1.7. In the UK, 3424 cases had been genomically confirmed by May 19, both imported and domesticallyacquired (Public Health England 20210507, Wise 20210521). Find a metaphor of an immunological B.1.671 landscape of hilly savannah with some distant mountains, featuring antelopes, hyenas and lions, at Tang J 2021.
Adverse events
As of now, the only side effect of concern seems to be an anaphylactic reaction which occurs very rarely (< 1:100,000) within minutes after receiving the vaccine. For a detailed discussion, see page 22.
Other side effects. Data on local and systemic reactions were collected with electronic diaries from participants in a reactogenicity subset of 8183 participants for 7 days after each vaccination. Local and systemic adverse events were reported more often by younger vaccine recipients (16 to 55 years of age) than by older vaccine recipients (older than 55 years of age) and more often after dose 2 than dose 1. Apart from pain at the injection site, the most commonly reported systemic events were fatigue and headache (see Tables 9 and 10). Most local and systemic reactions occur within the first 1 to 2 days after the injection and resolve within days. In some patients, axillary lymphadenopathy might indicate a robust vaccine-elicited immune response; it generally resolves within 10 days.
In comparison to these normal events, the incidence of serious adverse events was similar for tozinameran and placebo (0.6% and 0.5%, respectively).
| Table 9 – The BioNTech/Pfizer vaccine (Comirnaty™, Tozinameran; formerly BNT162b2): local and systemic reactions reported after the second injection of tozinameran or placebo (age group: 16-55 years) (FDA briefing document). See also Figure 2 of the paper by Polack et al. | ||
| Tozinameran
(Comirnaty™, |
Placebo | |
| Pain at injection site | 78% | 12% |
| Fever | 16% | 0% |
| Fatigue | 59% | 23% |
| Headache | 52% | 24% |
| Chills | 35% | 4% |
| Myalgia | 37% | 8% |
| Arthralgia | 22% | 5% |
| Table 10 – The BioNTech/Pfizer vaccine (Comirnaty™, Tozinameran; formerly BNT162b2): severe local and systemic reactions reported after the second injection of tozinameran or placebo (age group: 16-55 years) (FDA briefing document). | ||
| Tozinameran
(Comirnaty™, |
Placebo | |
| Pain at injection site | 1.2% | 0% |
| Fever >38.9° | 1.2% | 0.1% |
| Fatigue | 4.6% | 0.7% |
| Headache | 3.2% | 0.7% |
| Chills | 2.1% | 0% |
| Myalgia | 2.2% | 0.1% |
| Arthralgia | 1.0% | 0.2% |
COVID-19-vaccination–related adenopathy may sometimes be indistinguishable from malignant nodal involvement and must be excluded in patients with manifest or suspected cancer (Becker 2021, Tu W 2021). In one study, among 169 vacinees who were scanned a median of 52 days after the second vaccine dose, 29% had positive axillary uptake 7–10 weeks after second vaccination, divided to 42%, 31%, 25% and 19% on 7th, 8th, 9th and 10th weeks respectively (Eshet 2021).
Adolescents
In May 2021, the FDA authorized the BioNTech/Pfizer vaccine for adolescents 12 to 15 years of age after a Phase III trial had demonstrated 100% efficacy and robust antibody responses. Among 2260 adolescents enrolled in the United States, there were 18 cases of COVID-19 in the placebo group versus none in the vaccinated group (FDA 20210510). The safety profile was identical to adults, with slightly less reactions than adults. Children 12 to 15 years of age had almost twice the amount of antibodies than adults (Wallace 2021). This expansion of the emergency use authorization (EUA) will allow US middle school-aged students to get vaccinated before the beginning of the next school year.
Children 6 months to 11 years old
A global Phase I/II/III seamless trial to evaluate the safety, tolerability, and immunogenicity of the BioNTech/Pfizer vaccine in children 6 months to 11 years of age is under way. The trial will study three age groups: children aged 5 to 11 years, 2 to 5 years, and 6 months to 2 years (Pfizer 20210331). Results from this trial are expected in July for children five to twelve years old and in September for younger children. The evaluation of the trials is expected to take four to six weeks.
Pregnant women
In February 2021, Pfizer and BioNTech registered a Phase II/III trial to evaluate the safety, tolerability, and immunogenicity of their vaccine in approximately 4000 healthy pregnant women 18 years of age or older vaccinated at 24 to 34 weeks’ gestation (NCT04754594). In the meantime, the CDC recommends that pregnant women who become eligible may choose to get vaccinated (CDC 20210305).
Development
BioNTech and Pfizer have begun studying the safety and immunogenicity of a third dose of their vaccine to understand if a booster is sufficient to provide immunity against the new SARS-CoV-2 variants (Pfizer 20210225). In addition, the companies are planning a clinical study to evaluate a variant-specific vaccine with a modified mRNA sequence based on the B.1.351 lineage, first identified in South Africa.
Trivia
1,800,000,000 BioNTech/Pfizer doses. EU Commission President Ursula von der Leyen announces a €30+ billion contract for the purchase of 900 million doses of the BioNTech/Pfizer vaccine plus an option for another 900 million doses to be delivered by 2023. The contract includes agreements to adapt the vaccine to new virus variants and to assure production in the EU, both of the vaccine and of essential components (Reuters 20210508).
The Moderna vaccine
History and approval
In early February – after press releases, an emergency use authorization and the start of mass vaccinations – finally, the science behind the Moderna vaccine mRNA-1273 was published in an academic paper (Baden 2021). The Moderna vaccine has more than 90% efficacy at preventing COVID-19 illness, including severe disease. Moderate-to-severe systemic side effects, such as fatigue, myalgia, arthralgia, and headache, were noted in about 50% of participants in the mRNA-1273 group after the second dose. These side effects were transient, starting about 15 hours after vaccination and resolving in most participants by day 2, without sequelae. Antibodies elicited by the vaccine have been shown to persist through 6 months after the second dose (Doria-Rose 2021) – and will probably persist much longer.
The study by Baden et al. is the equivalent of the Polack study for the BioNTech/Pfizer vaccine. As of this writing (26 April), there is no real-world huge-scale data for the Moderna vaccine comparable to the data presented in the Dagan study for hundreds of thousand of individuals who received the BioNTech/Pfizer vaccine.
mRNA-1273, developed by Moderna, is a lipid nanoparticle–encapsulated nucleoside-modified messenger RNA (mRNA)–based vaccine that encodes the SARS-CoV-2 spike (S) glycoprotein stabilized in its prefusion conformation. The vaccine was approved on the basis of data from a Phase III trial which demonstrated that 100 μg taken four weeks apart conferred 94.5% protection against COVID-19 in persons 16 years of age or older (FDA EUA). Of 95 confirmed COVID-19 cases, 90 occurred in the placebo group and 5 in the vaccine group. Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical co-morbidities associated with high risk of severe COVID-19.
Previous studies had demonstrated that mRNA-1273 induced potent neutralizing antibody responses (Korber 2020, Widge 2020, Anderson 2020) to SARS-CoV-2 as well as CD8+ T cell responses, and protects against SARS-CoV-2 infection in mice (Corbett 2020) and non-human primates (Corbett 2020b). In early clinical trials, mRNA-1273 induced anti–SARS-CoV-2 immune responses in all participants, and no trial-limiting safety concerns were identified (Jackson 2020). Check also this article at https://www.nytimes.com/interactive/2020/health/moderna-covid-19-vaccine.html.
Efficacy against variants
There are to date no population-wide studies to assess the efficacy of the Moderna vaccine against the new SARS-CoV-2 variants B.1.1.7, B.1.351, P.1, B.1.429 and B.1.427.
In a neutralizing study of serum specimens obtained from 14 convalescent persons and from 49 recipients of the Moderna and the Novavax vaccine, B.1.429 (“California”) was approximately 2 to 3 times less sensitive to neutralization by convalescent serum and by serum samples obtained from vaccinated persons than the historical variant (Shen 2021). B.1.351 (“South Africa”) was approximately 9 to 14 times less sensitive.
Adverse events
As for now, the only side effect of concern seems to be an anaphylactic reaction which occurs very rarely (< 1:100,000) within minutes after receiving the vaccine. For a detailed discussion, see page 22.
A short discussion of other side effects:
- Side effects were transient, starting about 15 hours after vaccination and resolving in most participants by day 2, without sequelae (Baden 2020; see also Tables 11 and 12).
- With the exception of more frequent, generally mild to moderate reactogenicity in participants < 65 years of age, the safety profile of mRNA-1273 was generally similar across age groups, genders, ethnic and racial groups, and participants with or without medical co-morbidities.
- Several participants reported injection site reactions after day 7 that were characterized by erythema, induration, and often pruritis. Consultation with a dermatopathologist suggested that these were most likely dermal hypersensitivity reactions and were unlikely to represent a long-term safety concern.
- The rate of serious adverse events (SAEs) was low, and similar in both vaccine and placebo groups (around 1%). The most common SAEs in the vaccine group which were numerically higher than the placebo group were myocardial infarction (0.03%), cholecystitis (0.02%), and nephrolithiasis (0.02%), although the small numbers of cases of these events do not suggest a causal relationship (FDA Briefing). The most common SAEs in the placebo arm which were numerically higher than the vaccine arm, aside from COVID-19 (0.1%), were pneumonia (0.05%) and pulmonary embolism (0.03%). The incidence of serious adverse events was similar in the vaccine and placebo groups.
- There were three reports of facial paralysis (Bell’s palsy) in the vaccine group and one in the placebo group. There is insufficient information to determine a causal relationship with the vaccine.
| Table 11 – mRNA-1273: local and systemic reactions after the second injection of mRNA-1273 or placebo (18-64 years) (FDA Briefing). | |||
| mRNA-1273 | Placebo | ||
| Pain at injection site | 90% | 19% | |
| Lymphadenopathy | 16% | 4% | |
| Fever | 17% | 0% | |
| Fatigue | 68% | 25% | |
| Headache | 63% | 26% | |
| Chills | 48% | 6% | |
| Myalgia | 61% | 12% | |
| Arthralgia | 45% | 11% | |
| Table 12 – mRNA-1273: severe local and systemic reactions after the second injection of mRNA-1273 or placebo (18-64 years) (FDA Briefing). | |||
| mRNA-1273 | Placebo | ||
| Pain at injection site | 4.6% | 0.2% | |
| Lymphadenopathy | 0.4% | < 0.1% | |
| Fever | 1.6% | < 0.1% | |
| Fatigue | 10.6% | 0.8% | |
| Headache | 5.0% | 1.2% | |
| Chills | 1.5% | 0.1% | |
| Myalgia | 10.0% | 0.4% | |
| Arthralgia | 5.8% | 0.3% | |
With the Moderna vaccine (but not with the BioNTech vaccine), delayed localized cutaneous reactions near the injection site – pruritic, painful, and with edematous pink plaques (“COVID arm”) – have been described. They appear in a median of 7 days (range: 2-12 days) after the injection (Johnston 2021, n = 16). More than 70% of those who had a reaction to the first vaccine dose developed a similar reaction after the booster dose, generally sooner. Clinical and histopathologic findings suggest a self-limited delayed hypersensitivity reaction which is not a contraindication to subsequent vaccination.
Adolescents and children
In April, Moderna announced that a Phase II/III study of mRNA-1273 in adolescents ages 12-17 is fully enrolled with approximately 3000 participants in the US (Moderna 20210413). Results are expected by summer.
Another trial, a Phase II/III study of mRNA-1273 in children ages 6 months-11 years is currently enrolling in the US and Canada (target: 6750 participants) (Moderna 20210413). In Part 1 of this two-part, dose escalation study, children ages 2 years to less than 12 years will receive 50 μg or 100 μg. Children less than 2 years will receive 25 μg, 50 μg or 100 μg.
Development
Moderna has recently published a pre-print describing two updated versions of its vaccine: 1) mRNA-1273.351 which encodes for the S protein found in the B.1.351 lineage and 2) mRNA-1273.211 which comprises a 1:1 mix of mRNA-1273 and mRNA-1273.351. In Balb/c mice, both mRNA-1273.351 and mRNA-1273.211 increased neutralizing titers against against the B.1.351 variant first identified in South Africa (Wu K 2021). Both mRNA-1273.351 and mRNA-1273.211 are now being evaluated in pre-clinical challenge models and in Phase I/II clinical studies.
Moderna has also started a Phase I study to assess the safety and immunogenicity of mRNA-1283, a potential refrigerator stable mRNA vaccine that would simplify distribution and administration (Moderna 20210315). In future studies, mRNA-1283 could be evaluated for use as a booster dose for previously vaccinated or seropositive individuals.
Moderna is currently investigating various booster options for its mRNA-1273 vaccine:
- A single half-strength (50 µg) booster dose of the first-generation ‘standard’ mRNA-1273
- mRNA-1273.351, a second-generation vaccine candidate targeting the B.1.351 variant first detected (fdi) in South Africa
- mRNA-1273.211, a multivalent vaccine candidate which combines first generation mRNA-1273 and mRNA-1273.351 in a single vaccine
Initial data from a Phase II study would suggest that that a single 50 µg dose of mRNA-1273 or mRNA-1273.351 given as a booster approximately 6 to 8 months after the primary vaccination series increased neutralizing antibody titer responses against B.1.351 (fdi South Africa) and P.1 (fdi Brazil) (Moderna 20210505). A booster dose of mRNA-1273.351 achieved higher neutralizing antibody titers against B.1.351 than a booster dose of mRNA-1273. Adverse events following the third booster injection were generally comparable to those observed after the second dose of mRNA-1273 in previously reported Phase II and Phase III studies.
The AstraZeneca vaccine
History and approval
The development of the AstraZeneca vaccine Vaxzevria™ (formerly AZD1222, ChAdOx1 nCoV-19), developed by University of Oxford/AstraZeneca, has been plagued by turbid data, contract negotiations with EU, supply shortfalls and, lately, by a link to fatal venous sinus thromboses especially in younger vaccinees. In the US, a company press release about a 32,000-person study in the US, Peru and Chile (NCT D8110C00001) suggested a 76% efficacy against symptomatic SARS-CoV-2 infection occurring 15 days or more after receiving two doses given four weeks apart (AstraZeneca 20210325). This would be higher than the 59.5% reduction of symptomatic COVID-19 cases which was the basis for the authorization of use in the European Union (EMA 20210129).
The AstraZeneca vaccine uses replication-deficient chimpanzee adenovirus vector ChAdOx1, which contains the full-length, unmodified spike protein of SARS-CoV-2. Researchers involved in the development of ChAdOx1 nCoV-19 had previously published results from a Phase I/II trial showing that in ChAdOx1 vaccine recipients, T cell responses peaked on day 14, anti-spike IgG responses rose by day 28, and neutralizing antibody responses against SARS-CoV-2 were detected in > 90%. Adverse events such as fatigue, headache, and local tenderness commonly occurred, but there were no serious adverse events (Folegatti 2020). A multiplex cytokine profiling and intracellular cytokine staining analysis demonstrated that ChAdOx1 nCoV-19 vaccination induces a predominantly Th1-type response (Ewer 2020). In a Phase II/III trial ChAdOx1 nCoV-19 appeared to be better tolerated in older adults than in younger adults and had similar immunogenicity across all age groups after a booster dose (Ramasamy 2020, Andrew 2020). Finally, in December, the results from four randomized studies showed that ChAdOx1 had an efficacy of 62-90% (Voysey 2020, Knoll 2020). Public funding could have accounted for well over 90% of the funding towards the research and development of chimpanzee adenovirus-vectored vaccine (ChAdOx) technology at the University of Oxford for over two decades and, lately, of the Oxford-AstraZeneca vaccine (Cross 2021).
On December 30, UK regulatory authorities approved the vaccine (GOV.UK 20201230), followed a month later by the European Union (EMA 20210129). In February, WHO granted Emergency Use Listing (EUL) for active immunisation to prevent COVID-19 in individuals 18 years of age and older, including those over 65 (AstraZeneca 20210215). In March, COVAX began delivering millions of doses of the vaccine to 142 low- and middle-income countries as part of the effort to bring broad and equitable access to the vaccine (AstraZeneca 20210302). The first shipments were dispatched to Ghana, Cote D’Ivoire, the Philippines, Indonesia, Fiji, Mongolia and Moldova.
After a possible link between the AstraZeneca vaccine and rare, but life-threatening thromboses together with thrombocytopenia (vaccine-induced immune thrombotic thrombocytopenia, VITT; see page 13), it is unclear if the vaccine will be approved by the FDA. If it is approved, it is unclear if it will be used in the US – the country has plenty of alternative vaccines. On 26 April, a senior US administration official was quoted saying that there could be “up to 60 million doses of the AstraZeneca vaccine available to be shared with other countries in the next two months” (Collins 2021). In the European Union, some countries like Denmark have stopped using the AstraZeneca vaccine. The European Union has not canceled its existing orders of the AstraZeneca and Johnson & Johnson vaccines, but signaled it might not be going to be placing more (NYTimes 20210414). When future historians come to retell the story of the COVID-19 pandemic, they may observe that VITT helped settle the EU-UK dispute about insufficient AstraZeneca deliveries to the European Union.
Efficacy against variants
B.1.1.7 In Phase II/III vaccine efficacy studies in the UK, clinical efficacy of the AstraZeneca vaccine against symptomatic SARS-CoV-2 infection was slightly lower for B.1.1.7 lineages than for for non-B.1.1.7 lineages (70.4% vs 81.5%, respectively) (Emary 2021).
B.1.351. The AstraZeneca vaccine performed poorly in South Africa, as it offered no protection against mild-moderate COVID-19 (Madhi 2021). In early February, South Africa stopped plans for a rollout of 1 million doses of the vaccine.
P.1. No data.
Adverse events
As for possibly life-threatening thromboses together with thrombocytopenia after the administration of the AstraZeneca vaccine (EMA 20210407), see page 13.
Apart from this unusual and rare adverse event, the AstraZeneca vaccine is generally well tolerated (EMA 20210218, page 125). The most frequently reported solicited local adverse events (AEs) after any dose were tenderness (75.3% vs 54.2% in subjects who received a meningococcal ACWY vaccine) and pain (54.2% vs 35.4% in control). Severe local reactions were experienced by 0.8% of subjects.
The most frequently reported solicited systemic AEs were fatigue (62.3% vs 48.0% in control) and headache (57.5% vs 42.4% in control); other frequently reported systemic solicited AEs were muscle pain (48.6%), and malaise (44.2%). Pyrexia was reported in 9.2% participants who received any dose of the vaccine (vs 0.5% in control). Most of the systemic AEs following injection of the vaccine were mild or moderate. However, 9.3% of subjects experienced grade 3 systemic adverse events (malaise, chills, feverishness, etc.) (EMA 20210218, page 133).
Solicited local and systemic AEs were generally milder after the second dose than after the first dose of the vaccine.
Adolescents and children
In February, the University of Oxford announced the launch of the first study to assess the safety and immune responses of the AstraZeneca vaccine in children and young adults aged 6-17 years (Oxford University 20210212). The single-blind, randomised Phase II trial was to enrol 300 volunteers (240 would have received the AstraZeneca vaccine and the remainder a control meningitis vaccine). In early April, Oxford University announced that it was suspending the trial while British regulators investigated a potential blood clot link in adults. With British regulators recommending young adults 18 to 29 years old to be vaccinated with the BioNTech/Pfizer or the Moderna vaccine, the future of the AstraZeneca trial in childen and young adults aged 6-17 years is uncertain.
Development
In December, AstraZeneca and Gamaleya announced that they would combine their vaccines to see if the combination would deliver a stronger protection than either vaccine on its own. A Phase I trial was registered on Christmas Eve 2020.
AstraZeneca and Oxford University have started working on a 2nd generation of their vaccine which would be adapted to target SARS-CoV-2 variants with mutations similar to B.1.351 (Oxford University 20210207).
Future
The future role of the AstraZeneca product in the global COVID vaccine landscape is uncertain.
The Johnson & Johnson vaccine
History and approval
On 21 April, weeks after being authorized to be used in the USA (FDA 20210226) and Europe (EMA 20210311), the safety and efficacy data for the Johnson & Johnson (J&J) vaccine Ad26.COV2.S were finally published in a scientific journal (Sadoff 2021b). In a Phase III trial, the vaccine protected 66% of recipients against moderate to severe–critical COVID-19 and 85% against severe–critical COVID-19 one month after vaccination. Vaccine recipients who had breakthrough COVID-19 reported fewer and less severe symptoms than placebo recipients with COVID-19, which suggests that illness is milder after vaccination.
Earlier, results of a Phase I study (n = 25) had indicated that a single immunization with Ad26.COV2.S induced rapid binding and neutralization antibody responses as well as cellular immune responses (Stephenson 2021). In a later analysis, a single dose of Ad26.COV2.S was shown to elicit a strong humoral response in a majority of vaccine recipients (neutralizing antibodies in more than 90% of the participants in all age groups) and increasing antibody titers during 71 days of follow-up after the first dose. After two weeks, CD4+ T cell responses were detected in 76 to 83% (low dose vs high dose) among the 18 to 55 years old and and in 60 to 67% among those 65 years of age or older (Sadoff 2021). The CD8+ T cell responses were robust, but lower among the older participants.
Ad26.COV2.S is a recombinant replication-incompetent adenovirus type 26 (Ad26) vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen (Bos 2020). Its potency in eliciting protective immunity against SARS-CoV-2 infection was demonstrated in a non-human primate challenge model (Mercado 2020). Ad26.COV2.S induced robust neutralizing antibody responses and provided complete protection against a SARS-CoV-2 challenge in five out of six rhesus macaques and near-complete protection in one out of six macaques.
On March 12, the World Health Organization issued an Emergency Use Listing to Johnson & Johnson, accelerating its adoption by more countries (J&J 20210312).
Ad26.COV2.S is developed by the Janssen Pharmaceutical Companies of Johnson & Johnson.
Efficacy against variants
In South Africa, where the B.1.351 variant was already present at the time of the study, vaccine efficacy was 64% against moderate to severe–critical COVID-19 and and 82% against severe–critical COVID-19, one month after vaccination (Sadoff 2021b).
Adverse events
Cases of cerebral venous sinus thrombosis (CVST) concomitant with thrombocytopenia, first described for the AstraZeneca vaccine (see pages 13), have also been described after vaccination with the Johnson & Johnson vaccine (Muir 2021, Sadoff 2021, Shay 2021). On 20 April, the European Medicines Agency (EMA) found a ‘possible link’ between the Johnson & Johnson vaccine and thromboses ‘at unusual sites such as in veins in the brain (cerebral venous sinus thrombosis, CVST) and the abdomen (splanchnic vein thrombosis) and in arteries, together with low levels of blood platelets and sometimes bleeding’ (EMA 20210420). The cases reviewed were very similar to the cases that occurred with the COVID-19 vaccine developed by AstraZeneca, Vaxzevria. EMA also said the use of the Johnson & Johnson vaccine “at national level will take into account the pandemic situation and vaccine availability in individual Member States.”
On 23 April, the FDA and the CDC recommended resuming the use of the Johnson & Johnson vaccine (FDA 20210423) after a 10-day pause (FDA 20210413). The agencies used the pause to inform healthcare providers and clinicians of what they dubbed thrombosis-thrombocytopenia syndrome (TTS) and how to manage and recognize the adverse event. At that time, FDA and CDC were aware of 15 cases of TTS reported to the Vaccine Adverse Event Reporting system VAERS. All cases occurred in women between the ages of 18 and 59, with a median age of 37 years. Reports indicated symptom onset between 6 and 15 days after vaccination.
It has been suggested (Muir 2021) that the rare occurrence of vaccine-induced immune thrombotic thrombocytopenia could be related to adenoviral vector vaccines. This interpretation was swiftly contradicted by the manufacturer, pointing out the differences between the Johnson & Johnson and the AstraZeneca vaccine (Sadoff 2021).
Other adverse events. Apart from the very rare VITT/TTS events, the Johnson & Johnson vaccine is generally well tolerated (Shay 2021). The most frequent solicited adverse events (AEs) were fatigue, headache, myalgia, and injection site pain. The most frequent systemic AEs was fever. Systemic AEs were less common in participants 65 years of age or older than in those between the ages of 18 and 55 years (Sadoff 2021; see also FDA 20210226, page 39). The local and systemic reactions occurred on the day of immunization or the next day and generally resolved within 24 hours.
Vaccine providers should also be aware of anxiety-related events, including episodes of syncope which have been reported at a rate of 8.2 per 100,000 vaccinations with the Johnson & Johnson vaccine (for comparison: 0.05 per 100,000 influenza vaccines during the 2019/2020 season) (Hause 2021).
Pregnant women
In February, the company launched a trial for pregnant women with 400 participants (NCT04765384).
Development
In November 2020, Johnson & Johnson launched a second Phase III trial to evaluate the efficacy of two doses of Ad26.COV2.S in the prevention COVID-19, as compared to one dose of Ad26.COV2.S (NCT04614948).
Future
The European Union has not canceled its existing orders of the Johnson & Johnson vaccine, but signaled it might not be going to be placing more (NYTimes 20210414).
Vaccines approved outside the EU and the US
On 2 May 2021, four vaccines were approved outside the EU and the US in more than 10 countries (Table 13):
- The Gamaleya vaccine. Trade name: Sputnik V™ (formerly known as Gam-COVID-Vac)
- The Sinopharm vaccine, also known as BBIBP-CorV
- The Sinovac vaccine. Trade name: CoronaVac™ (formerly known as PiCoVacc)
- The Bharat vaccine. Trade name: Covaxin™ (formerly known as known as BBV152)
| Table 13. Vaccines approved outside the EU and the US in more than 10 countries | ||||
| Vaccine candidate Developers |
Vaccine platform | Type of candidate vaccine | Doses | Schedule |
| Gamaleya
Sputnik V |
Viral vector (Non-replicating) |
Adeno-based (rAd26-S+rAd5-S) |
2 | Day 0 + 21 |
| Sinopharm
BBIBP-CorV |
Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 | Day 0 + 21 |
| Sinovac
CoronaVac |
Inactivated virus | SARS-CoV-2 vaccine (inactivated) | 2 | Day 0 + 14 |
| Bharat
Covaxin |
Inactivated virus | Whole Virion Inactivated SARS-CoV-2 Vaccine (BBV152) | 2 | Day 0 + 28 |
The Gamaleya vaccine
Sputnik V (formerly known as Gam-COVID-Vac), developed by the Gamaleya Research Institute, is a combination of two genetically modified and replication-incompetent human common cold virus adenoviruses, Ad26 and Ad5, given 21 days apart, each carrying an S antigen of SARS-CoV-2. The administration of two different serotypes is expected to overcome any pre-existing adenovirus immunity (Lu S 2009, Barouch 2010). The vaccine can be stored at freezer temperatures of -18°C (0°F).
In early February 2021, preliminary results from an interim analysis of the Phase III Gam-COVID-Vac trial (n = 14,964 in the vaccine group and 4902 in the placebo group) were published in the Lancet. As for the primary outcome – the proportion of participants with PCR-confirmed COVID-19 – the trial reported an efficacy of 91.6% from 21 days after the first dose of the vaccine (on the day of dose 2). In the vaccine group, (16 (0.1%) participants had confirmed COVID-19, compared to 62 (1.3%) in the placebo group (Logunov 2021; see also the comment by Jones 2021). Most reported adverse events were mild (94%).
In a small study (n = 12), the vaccine has been reported to maintain neutralizing activity against B.1.1.7, with a moderate reduction against B.1.1.7 carrying the additional E484K (“Eek”) substitution. Against the B.1.351 variant (first detected in South Africa), as expected, only 1 out of 12 serum samples showed effective neutralization (Ikegame 2021).
Although the two-dose regimen with the Ad26 and Ad5 vectors is likely to remain the future standard for the Gamaleya vaccine, Gamaleya has started a single-dose trial with 110 participants (“Sputnik-Light”) (NCT04713488). The one-dose schedule could be proposed as a temporary solution for countries with high infection rates.
In December 2020, Gamaleya and AstraZeneca announced that they would combine their vaccines to test if the combination would deliver a stronger protection than either vaccine on its own (NCT04684446).
In March 2021, the European Medicines Agency (EMA) started a rolling review (EMA 20210304). In South America, on 26 April, Brazil’s health authority declared it would not recommend importing Gamaleya’s Sputnik V vaccine on grounds of “crucial questions” about safety and the manufacturing process (see the detailed discussion by Derek Lowe, 2021). On the same day, Brazil also announced that it had ordered 100 million doses of the BioNTech/Pfizer vaccine and 38 million doses of the Johnson & Johnson vaccine.
The hair-raising presidential approval in August 2020 before Phase III clinical trials had even begun, gave the vaccine a long-lasting credibility blow. The latest exercise in ridicule, claiming Sputnik to be ‘The first registered COVID-19 vaccine’, is equally embarassing.
The Sinovac vaccine
CoronaVac™ (formerly PiCoVacc) is an inactivated virus vaccine developed by Sinovac Biotech, a private Chinese company. In Brazil, CoronaVac is being developed in partnership with the Butantan Institute. In macaques, the vaccine provided partial or complete protection against SARS-CoV-2 challenge (Gao 2020). In a Phase I/II trial, CoronaVac was well-tolerated and moderately immunogenic in healthy adults aged 18–59 years. Most adverse reactions were mild, the most common symptom being injection site pain (Zhang Y 2020). In July 2020, the Chinese government approved CoronaVac for emergency use. In January 2021, the government of São Paulo, Brazil, announced the overall effectiveness of the Sinovac vaccine to be 50% in a study of 12,508 Brazilian health professionals. On 6 February, Sinovac announced that CoronaVac had been approved by the Chinese authorities. CoronaVac can be transported and refrigerated at 2–8 °C (36–46 °F).
In a real-world study in Chile, CoronaVac was shown to be 67% effective in preventing symptomatic infections after 14 days of the second dose. CoronaVac was also 85% effective in preventing hospitalization, 89% effective in preventing intensive care unit admission and 80% effective in preventing COVID-19-related death (Vergara 2021). The study, presented on 16 April by Chile’s Health Ministry, covered 10.5 million people, including 2.5 million who had received both doses of the vaccine and 1.5 million who had received a single dose during February and March 2021. In another real-world study of 21,652 Brazilian healthcare workers vaccinated between 18 January and 16 February with two doses of CoronaVac, the estimated effectiveness 2 and 3 weeks after the 2nd dose was 50.7% and 51.8%, respectively (de Faria 2021). Among 142 analyzed samples, 67 (47%) were variants of concern, mostly the P.1 strain. The discrepancy between the results of the two aforementioned studies is being discussed. Possible explanations include different dominant strains (in Brazil P.1 and P.2); different study populations (more exposed healthcare workers in the Brazilian study?); or more or less rigorous standards for defining a ‘case’ in trial participants.
In the setting of epidemic P.1 transmission, administration of one dose of CoronaVac has recently been estimated to be at least 35% effective against symptomatic SARS-CoV-2 infection (Hitchings 2021). In an in vitro analysis of 25 post-CoronaVac vaccination serum samples, B.1.351 has been shown to be more resistant to neutralization (by a factor of 2.5 to 3.3) than B.1.1.7 or the wild-type virus (Wang GL 2021).
In January 2021, according to a report of The New York Times (Wee SL 2021), Sinovac had sold more than 300 million doses, mostly to low- and middle-income countries. The contrast between a vaccine distributed by the hundreds of millions (CNA, 20 April), and the lack of published scientific data is disconcerting.
By early May, the vaccine had been approved in more than 20 countries, but not by FDA, EMA, or the Japanese or the Australian systems. On 4 May, the European Medicines Agency (EMA) announced that it had started a rolling review (EMA 20210504).
The Sinopharm vaccine
BBIBP-CorV is an inactivated virus vaccine developed by Sinopharm and the Beijing Institute of Biological Products, China. On 30 December 2020, the company announced that the vaccine had an efficacy of 79%. A day later, China’s health authorities approved the vaccine for general use (Davidson 2020, Wee SL 2021). BBIBP-CorV can be transported and stored at normal refrigerated temperatures.
Six months earlier, in June 2020, a Cell paper reported that BBIBP-CorV induced high levels of neutralizing antibodies titers in mice, rats, guinea pigs, rabbits, and non-human primates (cynomolgus monkeys and rhesus macaques). In rhesus macaques, a two-dose immunization also provided protection against SARS-CoV-2 intratracheal challenge, without detectable antibody-dependent enhancement of infection (Wang H 2020). In October 2020, results of a Phase I/II study showed that BBIBP-CorV was safe and well-tolerated in two age groups (18–59 years and ≥ 60 years) (Xia S 2021). On day 42, humoral responses had been induced in all vaccine recipients.
The B.1.1.7 variant showed little resistance to the neutralizing activity of vaccinee serum of 25 people 2 to 3 weeks after the second dose of BBIBP-CorV (Wang GL 2021). As anticipated (Liu Y 2021, Wang P 2021), the results were different for B.1.351 – 20 out of 25 serum samples showed complete or partial loss of neutralization. Another small study seems to contradict these findings, reporting 12 serum samples from recipients of the BBIBP-CorV vaccine which largely preserved neutralization of B.1.351 (Huang B 2021).
Sinopharm has yet to publish detailed results of their Phase III trial in peer-reviewed journals. On 7 May, WHO listed the vaccine for emergency use in people 18 years of age and older, giving the green light for the Sinopharm vaccine to be rolled out globally (WHO 20210507). WHO’s Emergency Use Listing (EUL) is also a prerequisite for COVAX vaccine supply. As for now, the vaccine has been approved in more than 40 countries.
The Bharat vaccine
Covaxin™ (formerly known as BBV152), developed by Bharat Biotech (Bharat Biotech, India) in collaboration with the Indian Council of Medical Research and the National Institute of Virology, is a whole virion inactivated SARS-CoV-2 vaccine, adjuvanted with Algel-IMDG (an imidazoquinoline molecule which is a toll-like receptor (TLR) 7/8 agonist, chemisorbed on alum [Algel]) (Ganneru 2021). In Syrian hamsters, Covaxin induced a potent humoral immune response, led to early clearance from the lower respiratory tract and protected the animals from pneumonia (Mohandas 2021). In rhesus macaques, too, the vaccine induced a strong immune response and protected the monkeys from pneumonia after infection with SARS-CoV-2, with complete viral clearance in nasal swab specimens 7 days post-infection (Yadav 2021).
In a Phase I/II trial (n = 375), the overall incidence rate of local and systemic adverse events was 14%-21% which seems to be lower than the rates for SARS-CoV-2 vaccines produced with other platforms such as mRNA (BioNTech/Pfizer, Moderna) or vector technology (AstraZeneca, Johnson & Johnson, Gamaleya) (Ella 2021). In a subsequent Phase III trial (n = 380), neutralizing antibody titres were similar to a panel of convalescent serum samples (Ella 2021b). Covaxin has also been reported to induce Th1-biased antibody responses with an elevated IgG2a/IgG1 ratio and increased levels of SARS-CoV-2-specific IFN-γ+ CD4+ T lymphocyte response (Ganneru 2021).
On 3 January, the Drugs Controller General of India (DCGI) approved the emergency use of Covaxin, making it India’s first vaccine against the pandemic, even though Phase III safety and efficacy clinical trials had not been completed. At that time, 22,500 of the 25,800 participants in a Phase III trial had been vaccinated (CTRI/2020/11/028976). On 21 April, Bharat Biotech announced that the second interim analysis of the Phase III study demonstrated a 78% vaccine efficacy in mild, moderate, and severe COVID-19 disease and a 70% efficacy against asymptomatic COVID-19 infection (Bharat 20210421).
Preliminary results suggest that Covaxin could be effective against B.1.1.7 (Sapkal 2021) and only slightly less effective against B.1.617 (Yadav 2021). These data need to be confirmed.
Bharat has yet to publish detailed results of their Phase III trial. As of 2 May, the vaccine has been approved in 14 countries.
Other vaccines
For information about other vaccines, refer to the excellent New York Times collection curated by Carl Zimmer, Jonathan Corum and Sui-Lee Wee:
- The Coronavirus Vaccine Tracker (Carl Zimmer, Jonathan Corum and Sui-Lee Wee) – Excellent overview of all vaccines in development, always up-to-date.
- How Nine Covid-19 Vaccines Work (Jonathan Corum and Carl Zimmer) – Almost 100 vaccines are in human trials. Find out how 9 of them work.
Coming vaccines
The Novavax vaccine
History
The Novavax vaccine ‘NVX-CoV2373’ is a recombinant “nanoparticle” vaccine (rSARS-CoV-2; sometimes also called a ‘protein[6] subunit vaccine’) composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant. The vaccine is produced by creating insect-infecting baculoviruses containing a gene for a modified SARS-CoV-2 spike protein which infects moth cells (the fall armyworm, Spodoptera frugiperda) in 2000 liter bioreactors (Wadman 2020). After extraction and chromatographical purification of the spike proteins, mixing with lipid particles and further co-formulation with the saponin-based adjuvant Matrix-M1 (Bangaru 2020, Tian JH 2021), NVX-CoV2373 is stored and stable at 2°-8°C (36°-46°F).
An early analysis revealed that the full-length immunogen was locked in a prefusion conformation (Bangaru 2020) and binds with high affinity to the ACE2 receptor. Early preclinical studies in mice and baboons revealed strong B and T cell responses to NVX-CoV2373 with no evidence of vaccine-associated enhanced respiratory disease (VAERS) (Tian JH 2021). In macaques, prime and booster immunization protected against SARS-CoV-2 replication in the nose and lungs, with no detectable replicating virus (sgRNA) in either upper or lower respiratory tracks (Guebre-Xabier 2020). Importantly, the authors of this paper also noted an absence of pulmonary pathology in the vaccinated animals.
A small Phase I/II clinical trial (Australia, n = 131) showed that the vaccine induced immune responses that exceeded levels seen in COVID-19 patients (Keech 2020). After the second vaccination neutralizing antibody responses were comparable to those seen in convalescent patients hospitalized with COVID-19.
Preliminary results from a Phase III trial in the UK (15,000 adult participants, with 27% percent being older than 65) suggest that the vaccine, administered as two injections 3 weeks apart, might be 96% effective against mild, moderate and severe disease caused by the historical SARS-CoV-2 strain (Novavax 20210311). Against variant SARS-CoV-2 strains, the Novavax vaccine:
- Maintains its efficacy more or less against the B.1.1.7 variant (first detected in England): 86.3% (95% CI: 71.3, 93.5) (Novavax 20210311)
- Shows 50% to 60% efficacy against the B.1.351 variant (depending on HIV serostatus). In a Phase II trial[7] in South Africa where most SARS-CoV-2 infections were caused by B.1.351 (Shinde 2021), efficacy was:
- 60.1% in HIV–negative participants
- 49.4% in HIV-positive participants
Of interest, in a preliminary efficacy analysis of the South African Phase II trial, the incidence of COVID-19 observed among participants who were SARS-CoV-2 seronegative at baseline was 5.3% (33 mild and 47 moderate cases among 1516 vaccinees), similar to the 5.2% incidence among SARS-CoV-2 seropositive vaccinees (14 mild and 21 moderate cases among 674 participants) (Shinde 2021), suggesting that “previous infection with historical viruses did not appear to reduce the risk of COVID-19 after subsequent infection with B.1.351 (Shinde 2021).
Efficacy against variants
Small in vitro studies of serum specimens from vaccinees suggest that B.1.1.7 (the variant first identified in [fii] England) and B.1.429 (fii California) are only slightly less sensitive to neutralization than the historical strain, whereas B.1.351 (fii South Africa) was found to be 9 to 14 times less sensitive (Shen X 2021, Shen X 2021b).
Adverse events
In a Phase II study in South Africa, adverse events generally subsisted within three days. The most common solicited systemic adverse events after both doses were pain at the injection site (more than one third of all vaccinees), headache (20 to 25%), muscle pain (17 to 20%), and fatigue (12 to 16) (Shinde 2021).
Children
The company has also expanded its Phase III trial to include up to 3000 children ages 12 to 17.
Development
Novavax has started a rolling review process of NVX-CoV2373 vaccine candidate with EMA, FDA, MHRA and Health Canada. However, because of regulatory and manufacturing problems, the Novavax vaccine is not expected to be authorized before July 2021 (Thomas 2021), with production reaching a peak only at the end of the year or later. This delay will impact a recent partnership with Gavi to supply 1.1 billion doses of the vaccine to low- and middle-income countries (Novavax 20210506). In April, financial analysts anticipated $33 billion in total sales 2021–2027 (GlobalData 20210408). The discrepancy between this forecast and the paucity of published scientific data is disconcerting.
Trivia
The Phase III PREVENT-19 trial (NCT04611802) in the US and Mexico was postponed several times as Novavax struggled to scale up its manufacturing capability.
Recently, the company announced that it would not be able to reach the initial production target of 150 million doses a month because of supply shortages including the bags used to grow the moth cells (Reuters 20210413).
In 34 years, Novavax has never won regulatory approval for any of a vaccine candidates.
Outlook
The immediate prospects of the COVID-19 pandemic are excellent, good or undetermined – depending on where you live.
Israel
The immediate prospects seem to be excellent for Israel (Balicier 2021). Soon, 60% of the total population will be fully vaccinated (Figure 2, green dots) which corresponds to more than 85% of the adult population as a third of the population is less than 16 years old. At the end of April, four months after the beginning of the vaccination campaign, the vast majority of adults had been vaccinated (see Table 14).
| Table 14. Israel, percentage of adults having received the first vaccine dose (by age group, end of April 2021): | |
| +90 years | 99.2% |
| 80-89 | 95.8% |
| 70-79 | 98.3% |
| 60-69 | 89.9% |
| 50-59 | 88.9% |
| 40-49 | 84.3% |
| 30-39 | 80.3% |
| 20-29 | 76.2% |
Despite an almost fully open economy since 7 March, the number of new daily cases has steadily declined ever since (Figure 2, red dashed line). From the peak in mid-January, the number of daily new cases has fallen by 98%, the number of new critically ill patients by 93%, and the number of daily deaths by 87% (Figure 3). Of interest, this effect was achieved with 60% of the population vaccinated (not 70%, 80% or 90%!).
Israel was the theater of the pivotal Dagan study (Dagan 2021; see also Efficacy, page 7).
Figure 2. SARS-CoV-2 cases in Israel, 10 May 2021. Impact of mass vaccination on the pandemic. The rolling 7-day average of new SARS-CoV-2 cases is shown in red (left vertical axis), the rolling 7-day average of deaths as the solid black line (right vertical axis). The percentage of people that have received at least one vaccine dose is shown in dotted green. The percentage of people that have been fully vaccinated is shown in solid green. The evolution of daily new cases and deaths was influenced by lockdown measures, transmissibility of circulating viruses and the vaccination campaign.
Figure 3. Israel, 25 April 2021. After a massive vaccination campaign, the number of daily new cases fell by 98% (blue), the number of new critically ill patients by 97% (orange), and the number of daily deaths by 99% (green). Source and copyright: Eran Segal, 25 April, https://bit.ly/3nneHgN.
During the first 112 days (December 2020 through April 2021), Israel’s vaccination campaign has been estimated to have averted at least 150,000 SARS-CoV-2 infections, 17,000 severe and critical hospitalizations, and 5,000 deaths (91% of these averted among individuals ≥ 65 years of age) (Haas 2021b).
United States
For the US, the prospects are good. Although some states saw increasing numbers of daily new cases in what could have been the beginning of a fourth wave, the impressive US vaccination campaign was able to control the epidemic. In May, some Americans started discovering pre-COVID-19 freedom. The new recommendations published on 13 May by the CDC (CDC 20210513):
- If you are fully vaccinated, you can resume activities that you did prior to the pandemic. In general, people are considered fully vaccinated:
- 2 weeks after their second dose in a 2-dose series, such as the Pfizer or Moderna vaccines, or
- 2 weeks after a single-dose vaccine, such as Johnson & Johnson’s Janssen vaccine
- Fully vaccinated people can resume activities without wearing a mask or physically distancing, except where required by federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
- If you haven’t been vaccinated yet, find a vaccine.
Find more details at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html.
The last obstacle before achieving community-wide immunity and a return to some pre-COVID-19 life? Vaccine hesitancy!
France
On 19 May, the French government ordered the progressive easing of restriction measures and – unknowingly? – an informative experiment: will an aggressive vaccination campaign at a time of relatively low vaccination coverage (only 15% of the population was fully vaccinated and an additional 17% had received the first vaccine dose) be sufficient to end the national epidemic? Or will there be a fourth wave, particularly among young, non-vaccinated people? For comparison, Israel opened most of its economy after a two-month lockdown on 7 March, when 57.1% and 43.8% of the population had received one or two doses, respectively (Figure 4). Les jeux ne sont pas encore faits.
Figure 4. France in May 2021. “Daily new confirmed COVID-19 cases per million people”. Published online at OurWorldInData.org – accessed 24 May 2021.
In check
Figure 3 (see above) provides a glimpse of a world where SARS-CoV-2 is being kept in check. Over the next months, we will address the following questions:
- Are COVID vaccines effective in children from 6 months to 11 years of age?
- Can vaccine doses from different manufacturers be used? (First dose with vaccine A, second dose with vaccine B?) Preliminary data suggest that the second dose of heterologous vaccine schedules (BioNTech/Pfizer + AstraZeneca; or AstraZeneca + BioNTech/Pfizer) might induce more frequent, generally mild adverse events (Shaw 2021). Data about the primary immunological outcome are expected in June.
- Which percentage of vaccinated people will have symptomatic or asymptomatic SARS-CoV-2 infection within 6, 12, 18 or 24 months after vaccination?
- Do vaccines prevent long COVID in vaccinated people who develop symptomatic SARS-CoV-2 infection?
- Are fully vaccinated people less likely to transmit SARS-CoV-2 to others if they get infected?
- To what extent will the B.1.351 and P.1 variants escape vaccine-induced immunity? If yes, will ‘updated’ versions of existing vaccines, especially the mRNA vaccines, be available soon?
- Will the efficacy of these second-generation mRNA booster vaccines be diminished by ‘antigenic sin’? Probably not.
- Will these second-generation vaccines have acceptable side effects?
Two pieces of good news are coming in from the variants front. The BioNTech vaccine has been shown to be effective against B.1.351 (first detected in South Africa). In Qatar, in a real-world test, the effectiveness against any B.1.351 infection was 75%, approximately 20 percentage points lower than the effectiveness reported in studies from Israel (Dagan 2021, Haas 2021); however, effectiveness against severe, critical, or fatal disease was well over 90% (Abu-Raddad 2021). In vitro data had already suggested that the SARS-CoV-2 vaccines would retain activity against the B.1.351 (first detected in South Africa) and P.1 (Brazil) (Moyo-Gwete 2021, Liu Y 2021, Lustig 2021, Reynolds 2021).
In a more distant future, we will appreciate how SARS-CoV-2 contributed to advances in medicine. One of the first achievements could be a pan-coronavirus vaccine. Some ground-breaking research has recently been published. First, a characterization of almost 3000 S-reactive T cell clones from 34 COVID-19 individuals revealed an immunodominant S346-365 region within the receptor-binding domain (RBD) that is highly conserved among zoonotic and human sarbecoviruses, including SARS-CoV-2 and its variants of concern. The S346-365 region was recognized by 94% of individuals and by 33% of the clones (Low 2021). Second, the vaccination of macaques with a pan-coronavirus mRNA nanoparticle vaccine elicited cross-neutralizing antibody responses against batCoVs, SARS-CoV-1, SARS-CoV-2, and SARS-CoV-2 variants B.1.1.7, P.1, and B.1.351 (Saunders 2021). This is just the first glimpse of new and momentous developments to come.
References
Abbasi J. COVID-19 and mRNA Vaccines-First Large Test for a New Approach. JAMA. 2020 Sep 3. PubMed: https://pubmed.gov/32880613. Full text: https://doi.org/10.1001/jama.2020.16866
Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021 Feb;26(6):2100096. PubMed: https://pubmed.gov/33573712. Full-text: https://doi.org/10.2807/1560-7917.ES.2021.26.6.2100096
Abu-Raddad LJ, Chemaitelly H, Butt AA; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021 May 5. PubMed: https://pubmed.gov/33951357. Full-text: https://doi.org/10.1056/NEJMc2104974
Aeberhardt C, Gatinois C. Comment Sanofi s’est retrouvé distancé dans la course au vaccin contre le Covid-19. Le Monde 2021, published 11 January. Full text : https://www.lemonde.fr/economie/article/2021/01/11/covid-19-le-destin-contrarie-du-vaccin-de-sanofi_6065825_3234.html
Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020 Oct 22;371:m3939. PubMed: https://pubmed.gov/33093056. Full text: https://doi.org/10.1136/bmj.m3939
Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016 Sep;12(9):2351-6. PubMed: https://pubmed.gov/27269431. Full text: https://doi.org/10.1080/21645515.2016.1177688
Alter G, Gorman M, Patel N, et al. Collaboration between the Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M™ vaccination. Res Sq. 2021 Feb 15:rs.3.rs-200342. PubMed: https://pubmed.gov/33619473. Full-text: https://doi.org/10.21203/rs.3.rs-200342/v1
Alter G, Seder R: The Power of Antibody-Based Surveillance. N Engl J Med 2020, published 1 September. Full text: https://doi.org/10.1056/NEJMe2028079.
Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med, December 17 2020; 383:2427-2438. Full text: https://doi.org/10.1056/NEJMoa2028436
Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet 2020, published 18 November. Full text: https://doi.org/10.1016/S0140-6736(20)32481-8
ANSM 20210416. Point de situation sur la surveillance des vaccins contre la COVID-19 – Période du 02/04/2021 au 08/04/2021. L’Agence nationale de sécurité du médicament et des produits de santé (ANSM) 2021, published 16 April. Full text: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-02-04-2021-au-08-04-2021
ANSM 20210423. Point de situation sur la surveillance des vaccins contre la COVID-19 – Période du 09/04/2021 au 15/04/2021. L’Agence nationale de sécurité du médicament et des produits de santé (ANSM) 2021, published 23 April. Full text: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-09-04-2021-au-15-04-2021
ANSM 20210517. Point de situation sur la surveillance des vaccins contre la COVID-19 – Période du 30/04/2021 au 06/05/2021. L’Agence nationale de sécurité du médicament et des produits de santé (ANSM) 2021, published 17 May. Full text: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-30-04-2021-au-06-05-2021
Arnold C. How computational immunology changed the face of COVID-19 vaccine development. Nat Med. 2020 Jul 15. PubMed: https://pubmed.gov/32669667. Full text: https://doi.org/10.1038/d41591-020-00027-9
AstraZeneca 20210215. AstraZeneca COVID-19 vaccine authorised for emergency use by the World Health Organization. AstraZeneca press release 2021, published 15 February. Full text: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/astrazeneca-covid-19-vaccine-authorised-for-emergency-use-by-the-world-health-organization.html
AstraZeneca 20210302. AstraZeneca COVID-19 vaccine authorised for emergency use by the World Health Organization. AstraZeneca press release 2021, published 2 March. Full text: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/astrazeneca-advances-mass-global-rollout-of-covid-19-vaccine-through-covax.html
AstraZeneca 20210325. AZD1222 US Phase III primary analysis confirms safety and efficacy. AstraZeneca 2021, published 25 March. Full text: https://www.astrazeneca.com/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403-416. PubMed: https://pubmed.gov/33378609. Full text: https://doi.org/10.1056/NEJMoa2035389
Bailly B, Guilpain L, Bouiller K, et al. BNT162b2 mRNA vaccination did not prevent an outbreak of SARS COV-2 variant 501Y.V2 in an elderly nursing home but reduced transmission and disease severity. Clin Infect Dis 2021, published 16 May. Full text: https://doi.org/10.1093/cid/ciab446
Balicier RD, Ohana R. Israel’s COVID-19 endgame. Science 2021, published 14 May. Full text: https://science.sciencemag.org/content/372/6543/663
Ball P. The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature 2020, published 18 December. Full text: https://www.nature.com/articles/d41586-020-03626-1
Bangaru S, Ozorowski G, Turner HL, et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science. 2020 Nov 27;370(6520):1089-1094. PubMed: https://pubmed.gov/33082295. Full-text: https://doi.org/10.1126/science.abe1502
Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011 Jul 18;29(32):5203-9. PubMed: https://pubmed.gov/21619905. Full-text: https://doi.org/10.1016/j.vaccine.2011.05.025
Bar-Zeev N, Moss WJ. Encouraging results from phase 1/2 COVID-19 vaccine trials. Lancet. 2020 Aug 15;396(10249):448-449. PubMed: https://pubmed.gov/32702300. Full text: https://doi.org/10.1016/S0140-6736(20)31611-1
Baum A, Ajithdoss D, Copin R, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020b, published 9 October. Full-txt: https://doi.org/10.1126/science.abe2402
Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020 Aug 21;369(6506):1014-1018. PubMed: https://pubmed.gov/32540904. Full text: https://doi.org/10.1126/science.abd0831
Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021 Apr 14:S0140-6736(21)00872-2. PubMed: https://pubmed.gov/33864750. Full text: https://doi.org/10.1016/S0140-6736(21)00872-2
Becker AS, Perez-Johnston R, Chikarmane SA, et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology. 2021 Feb 24:210436. PubMed: https://pubmed.gov/33625298. Full-text: https://doi.org/10.1148/radiol.2021210436
Behr MA, Divangahi M, Schurr E. Lessons from BCG for SARS-CoV-2 vaccine candidates. J Infect Dis. 2020 Nov 30:jiaa637. PubMed: https://pubmed.gov/33252679. Full text: https://doi.org/10.1093/infdis/jiaa637
Benedict C, Cedernaes J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir Med. 2021 Mar 12:S2213-2600(21)00126-0. PubMed: https://pubmed.gov/33721558. Full text: https://doi.org/10.1016/S2213-2600(21)00126-0
Bharat 20210421. Bharat Biotech and ICMR Announce Interim Results from Phase 3 trials of COVAXIN®; Demonstrates overall Interim Clinical Efficacy of 78% and 100% efficacy against Severe COVID-19 disease. Bharat Biotech 2021, published 21 April. Full text: https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf
Bingham K. Plan now to speed vaccine supply for future pandemics. Nature. 2020 Oct;586(7828):171. PubMed: https://pubmed.gov/33024331. Full text: https://doi.org/10.1038/d41586-020-02798-0
Bingham K. Plan now to speed vaccine supply for future pandemics. Nature. 2020 Oct;586(7828):171. PubMed: https://pubmed.gov/33024331. Full text: https://doi.org/10.1038/d41586-020-02798-0
Biopharma. Hunting for antibodies to combat COVID‑19. Biopharma dealmakers 2020, published 1 September. Full text: https://www.nature.com/articles/d43747-020-01115-y
Blain H, Tuaillon E, Gamon L, et al. Spike Antibody Levels of Nursing Home Residents With or Without Prior COVID-19 3 Weeks After a Single BNT162b2 Vaccine Dose. JAMA. 2021 Apr 15. PubMed: https://pubmed.gov/33856406. Full-text: https://doi.org/10.1001/jama.2021.6042
Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011 Dec;85(23):12201-15. PubMed: https://pubmed.gov/21937658. Full text: https://doi.org/10.1128/JVI.06048-11
Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020 Sep 28;5:91. PubMed: https://pubmed.gov/33083026. Full text: https://doi.org/10.1038/s41541-020-00243-x
Braun J, Loyal L, Frentsch, M, et al. Presence of SARS-CoV-2-reactive T cells in COVID-19 patients and healthy donors. medRxiv 22 April 2020. Full text: https://doi.org/10.1101/2020.04.17.20061440 (accessed 2 June 2020)
Burki T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit Health. 2020 Oct;2(10):e504-e505. PubMed: https://pubmed.gov/32984795. Full text: https://doi.org/10.1016/S2589-7500(20)30227-2
Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature 2021, published 8 January. Full text: https://www.nature.com/articles/d41586-021-00031-0
Callaway E. Dozens to be deliberately infected with coronavirus in UK ‘human challenge’ trials. Nature 2020, published 20 October. Full text: https://www.nature.com/articles/d41586-020-02821-4
Callow KA, Parry HF, Sergeant M, Tyrrell DA The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990 Oct;105(2):435-46. https://pubmed.gov/2170159. Full text: https://pmlegacy.ncbi.nlm.nih.gov/pubmed/2170159
Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018 Oct;16(10):1918-1931. PubMed: https://pubmed.gov/29923367. Full text: https://doi.org/10.1111/jth.14210
Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004 Sep;2(9):695-703. PubMed: https://pubmed.gov/15372080. Full text: https://doi.org/10.1038/nrmicro974
Castells MC, Phillips EJ. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med 2020, published 30 December. Full text: https://doi.org/10.1056/NEJMra2035343
Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39(1-3):225-39. PubMed: https://pubmed.gov/17917067. Full text: https://doi.org/10.1007/s12026-007-0071-6
Cavanaugh AM, Fortier S, Lewis P. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program — Kentucky, March 2021. MMWR 2021, published 21 April. Full text: https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e2.htm
CDC 20201231. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. Vaccines & Immunizations 2020, last reviewed: December 31, 2020. Full text: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/anaphylaxis-management.html – Accessed 3 January 2021.
CDC 2021 NCV. New COVID-19 Variants. Centers for Disease Control 2021, updated 3 January. Full text: https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html – Accessed 12 January 2021.
CDC 2021 V. Emerging SARS-CoV-2 Variants. Centers for Disease Control 2021, updated 3 January. Full text: https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html – Accessed 11 January 2021.
CDC 20210106. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. ePub: 6 January 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7002e1
CDC 20210218. Risk for COVID-19 Infection, Hospitalization, and Death By Age Group. CDC 2021, 18 February (accessed 31 March). Full text: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html
CDC 20210303. Interim Considerations: Preparing for the Potential Management of Anaphylaxis after COVID-19 Vaccination. CDC 2021, updated 3 March (accessed 31 March). Full text: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html
CDC 20210305. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. CDC 2021, updated 5 March (accessed 31 March). Full text: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
CDC 20210513. When You’ve Been Fully Vaccinated. CDC 2021, published 13 May, accessed 15 May. Full text: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021 Mar 29. PubMed: https://pubmed.gov/33780970. Full text: https://doi.org/10.1038/s41586-021-03471-w
Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol. 2014 Oct;88(19):11034-44. PubMed: https://pubmed.gov/25056892. Full text: https://doi.org/10.1128/JVI.01505-14
Chapin-Bardales J, Gee J, Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. 2021 Apr 5. PubMed: https://pubmed.gov/33818592. Full text: https://doi.org/10.1001/jama.2021.5374
Chen J, Subbarao K. The Immunobiology of SARS*. Annu Rev Immunol. 2007;25:443-72. PubMed: https://pubmed.gov/17243893. Full text: https://doi.org/10.1146/annurev.immunol.25.022106.141706
Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021 May;9(5):e598-e609. PubMed: https://pubmed.gov/33705690. Full text: https://doi.org/10.1016/S2214-109X(21)00026-7
Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Sig Transduct Target Ther 5, 180 (2020). Full text: https://doi.org/10.1038/s41392-020-00301-9
Chun S, Chung CR, Ha YE, et al. Possible Transfusion-Related Acute Lung Injury Following Convalescent Plasma Transfusion in a Patient With Middle East Respiratory Syndrome. Ann Lab Med. 2016 Jul;36(4):393-5. PubMed: https://pubmed.gov/27139619. Full text: https://doi.org/10.3343/alm.2016.36.4.393
Cines DB, Bussel JB. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. N Engl J Med 2021, published 16 April. Full text: https://www.nejm.org/doi/full/10.1056/NEJMe2106315
Cohen J. Designer antibodies could battle COVID-19 before vaccines arrive. Science 2020, published 4 August. Full text: https://www.sciencemag.org/news/2020/08/designer-antibodies-could-battle-covid-19-vaccines-arrive
Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA Network 2021, published 13 May. Full text: https://jamanetwork.com/journals/jama/fullarticle/2780202
Collins K, Liptak K, Vazquez M. US to begin sharing AstraZeneca coronavirus vaccine doses soon. CNN 2021 published 26 April. Full text: https://edition.cnn.com/2021/04/26/politics/astrazeneca-vaccines-us-share/index.html
Cookson C. UK to test vaccines on volunteers deliberately infected with Covid-19. Financial Times 2020, published 23 September. Full text: https://www.ft.com/content/b782f666-6847-4487-986c-56d3f5e46c0b
Corbett KS, Edwards DK, Leist SR et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, published 5 August. Full text: https://doi.org/10.1038/s41586-020-2622-0
Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med 2020b, published 28 July. Full text: https://doi.org/10.1056/NEJMoa2024671
Cott E, deBruyn E, Corum J. How Pfizer Makes Its Covid-19 Vaccine. The New York Times 2021, published 28 April. Full text: https://www.nytimes.com/interactive/2021/health/pfizer-coronavirus-vaccine.html
Cross S, Rho Y, Reddy H, et al. Who funded the research behind the Oxford-AstraZeneca COVID-19 vaccine? Approximating the funding to the University of Oxford for the research and development of the ChAdOx vaccine technology. medRxiv 2021, posted 10 April. Full text: https://doi.org/10.1101/2021.04.08.21255103
Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018 Nov 27;2(22):3360-3392. PubMed: https://pubmed.gov/30482768. Full text: https://doi.org/10.1182/bloodadvances.2018024489
CureVac 20210107. CureVac and Bayer join forces on COVID-19 vaccine candidate CVnCoV – joint press release. CureVac and Bayer 2021, 7 January. Full text: https://www.curevac.com/en/2021/01/07/curevac-and-bayer-join-forces-on-covid-19-vaccine-candidate-cvncov/
CureVac 20210203. GSK and CureVac to develop next generation mRNA COVID-19 vaccines – joint press release. CureVac and GSK, 3 February. Full text: https://www.curevac.com/en/2021/02/03/gsk-and-curevac-to-develop-next-generation-mrna-covid-19-vaccines
CureVac 20210304. CureVac and Novartis Sign Initial Agreement on Manufacturing of COVID-19 Vaccine Candidate, CvnCoV. CureVac and Novartis, 4 March. Full text: https://www.curevac.com/en/2021/03/04/curevac-and-novartis-sign-initial-agreement-on-manufacturing-of-covid-19-vaccine-candidate-cvncov
CureVac 20210513. Second-Generation COVID-19 Vaccine Candidate, CV2CoV, Demonstrates High Immunogenicity Against Virus Variants in Preclinical Study. CureVac 2021, 13 May. Full text: https://www.curevac.com/en/2021/05/13/second-generation-covid-19-vaccine-candidate-cv2cov-demonstrates-high-immunogenicity-against-virus-variants-in-preclinical-study
Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. NEJM February 24, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2101765
Dagotto G, Yu J, Barouch DH. Approaches and Challenges in SARS-CoV-2 Vaccine Development. Cell Host Microbe 2020, published 10 August. Full text: https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(20)30455-8
Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063. PubMed: https://pubmed.gov/33408181. Full text: https://doi.org/10.1126/science.abf4063 – See also the press article by Mandavilli A. Immunity to the Coronavirus May Last Years, New Data Hint. The New York Times 2020, published 17 November. Full text: https://www.nytimes.com/2020/11/17/health/coronavirus-immunity.html.
Davidson 2020. China approves Sinopharm Covid-19 vaccine for general use. The Guardian 2020, published 31 December. Full text: https://www.theguardian.com/world/2020/dec/31/china-approves-sinopharm-covid-19-vaccine-for-general-use
de Faria E, Guedes AR, Oliveira MS, et al. Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW) – preliminary report. medRxiv 2021, posted 15 April. Full text: https://doi.org/10.1101/2021.04.12.21255308
De Souza WM, Amorm MR, Sesti-Costa R, et al. Levels of SARS-CoV-2 Lineage P.1 Neutralization by Antibodies Elicited after Natural Infection and Vaccination. Lancet Preprints 2021, posted 1 March. Full text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3793486
Deming ME, Michael NL, Robb M, et al. Accelerating Development of SARS-CoV-2 Vaccines — The Role for Controlled Human Infection Models. NEJM July 1, 2020. Full text: https://doi.org/10.1056/NEJMp2020076. Full text: https://www.nejm.org/doi/full/10.1056/NEJMp2020076
Dolgin E. How COVID unlocked the power of RNA vaccines. Nature 2021, published 12 January. Full text: https://www.nature.com/articles/d41586-021-00019-w
Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 Apr 6. PubMed: https://pubmed.gov/33822494. https://www.nejm.org/doi/10.1056/NEJMc2103916
Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N Engl J Med. 2017 Jun 22;376(25):2427-2436. PubMed: https://pubmed.gov/28636855. Full-text: https://doi.org/10.1056/NEJMoa1608862
ECDC 20201220a. Threat Assessment Brief: Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. European Centre for Disease Prevention and Control 2020, published 20 December. Full text: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-rapid-increase-sars-cov-2-variant-united-kingdom
ECDC 20201220b. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. European Centre for Disease Prevention and Contro 2020, published 20 December. Full text: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf
Edara VV, Lai L, Sahoo M, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv 2021, posted 10 May. Full text: https://doi.org/10.1101/2021.05.09.443299
Eguia R, Crawford KHD, Stevens-Ayers T, et al. A human coronavirus evolves antigenically to escape antibody immunity. bioRxiv 2020, posted 18 December. Full text: https://www.biorxiv.org/content/10.1101/2020.12.17.423313v1
Elias C, Sekri A, Leblanc P, Cucherat M, Vanhems P. The incubation period of COVID-19: A meta-analysis. Int J Infect Dis. 2021 Mar;104:708-710. PubMed: https://pubmed.gov/33548553. Full-text: https://doi.org/10.1016/j.ijid.2021.01.069
Ella R, Vadrevu KM, Jogdand H, et al. A Phase 1: Safety and Immunogenicity Trial of an Inactivated SARS-CoV-2 Vaccine-BBV152. medRxiv 2020, posted 15 December. Full text: https://doi.org/10.1101/2020.12.11.20210419
Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021 May;21(5):637-646. PubMed: https://pubmed.gov/33485468. Full-text: https://doi.org/10.1016/S1473-3099(20)30942-7
Ellis-Petersen 20210421. ‘The system has collapsed’: India’s descent into Covid hell. The Guardian 2021, published 21 April. Full text: https://www.theguardian.com/world/2021/apr/21/system-has-collapsed-india-descent-into-covid-hell
EMA 20210129. EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU. European Medicines Agency 2021, published 29 January. Full text: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu
EMA 20210212. EMA starts rolling review of CureVac’s COVID-19 vaccine (CVnCoV). European Medicines Agency 2021, published 12 February. Full text: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-curevacs-covid-19-vaccine-cvncov
EMA 20210218. Vaxzevria (previously COVID-19 Vaccine AstraZeneca) : EPAR – Public assessment report. EMA/94907/2021. European Medicines Agency 2021, published 18 February. Full text: https://www.ema.europa.eu/documents/assessment-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-public-assessment-report_en.pdf – Download from https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca
EMA 20210304. EMA starts rolling review of the Sputnik V COVID-19 vaccine. European Medicines Agency 2021, published 4 March. Full text: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-sputnik-v-covid-19-vaccine
EMA 20210311. EMA recommends COVID-19 Vaccine Janssen for authorisation in the EU. European Medicines Agency 2021, published 11 March. Full text: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-janssen-authorisation-eu + https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen
EMA 20210407. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. EMA 2021, published 7 April. Full text: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood
EMA 20210420. COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. European Medicines Agency 2021, published 20 April. Full text: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood
EMA 20210423. AstraZeneca’s COVID-19 vaccine: benefits and risks in context. European Medicines Agency 2021, published 23 April (accessed 23 April). Full text: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context + Graphic representation of the findings: https://www.ema.europa.eu/documents/chmp-annex/annex-vaxzevria-art53-visual-risk-contextualisation_en.pdf
EMA 20210504. EMA starts rolling review of COVID-19 Vaccine (Vero Cell) Inactivated. European Medicines Agency 2021, 4 May 2021. Full text: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-covid-19-vaccine-vero-cell-inactivated
EMA 20210517. More flexible storage conditions for BioNTech/Pfizer’s COVID-19 vaccine. European Medicines Agency 2021, published 17 May. Full text: https://www.ema.europa.eu/en/news/more-flexible-storage-conditions-biontechpfizers-covid-19-vaccine
Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021 Apr 10;397(10282):1351-1362. PubMed: https://pubmed.gov/33798499. Full text: https://doi.org/10.1016/S0140-6736(21)00628-0
ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021 Jan 1;36(1):87-94. PubMed: https://pubmed.gov/33340043. Full-text: https://doi.org/10.1093/ndt/gfaa314
Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology. 2021 Apr 27:210886. PubMed: https://pubmed.gov/33904778. Full-text: https://doi.org/10.1148/radiol.2021210886
Ewer KJ, Barrett JR, Belij-Rammerstorfer S. et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med December 18, 2020. Full text: https://doi.org/10.1038/s41591-020-01194-5
Faria NR, Claro IM, Candido D, et al. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological.org 2021, posted 12 January. Full text: https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586
Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. MedRxiv 2021, posted 3 March. Full text: https://doi.org/10.1101/2021.02.26.21252554
FDA 20210226. Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021 – FDA Briefing Document. FDA 2021, published 26 February. Full text: https://www.fda.gov/media/146217/download + https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine
FDA 20210413. Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. FDA 2021, published 13 April. Full text: https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine
FDA 20210423. FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review. FDA 2021, published 23 April. Full text: https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough
FDA 20210510. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic. FDA 2021, published 10 May. Full text: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use
Felter C. How Dangerous Are New COVID-19 Strains? Council on Foreign Relation 2021, published 7 January. Full text: https://www.cfr.org/in-brief/how-dangerous-are-new-covid-19-strains
Ferreira I, Datir R, Papa G, et al. SARS-CoV-2 B.1.617 emergence and sensitivity to vaccine-elicited antibodies. bioRxiv 2021, posted 9 May. Full text: https://doi.org/10.1101/2021.05.08.443253
Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467-478. PubMed: https://pubmed.gov/32702298. Full text: https://doi.org/10.1016/S0140-6736(20)31604-4
Gallagher T, Lipsitch M. Postexposure Effects of Vaccines on Infectious Diseases. Epidemiol Rev. 2019 Jan 31;41(1):13-27. PubMed: https://pubmed.gov/31680134. Full-text: https://doi.org/10.1093/epirev/mxz014
Ganneru B, Jogdand H, Daram VK, et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. iScience. 2021 Apr 23;24(4):102298. PubMed: https://pubmed.gov/33723528. Full-text: https://doi.org/10.1016/j.isci.2021.102298
Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 Jul 3;369(6499):77-81. PubMed: https://pubmed.gov/32376603. Full text: https://doi.org/10.1126/science.abc1932
GlobalData 20210408. Novavax’s COVID-19 vaccine is forecast to generate $1.8bn sales in 2021, says GlobalData. GlobalData 2021, published 8 April. Full text: https://www.globaldata.com/novavaxs-covid-19-vaccine-forecast-generate-1-8bn-sales-2021-says-globaldata
Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid-19 in people who report allergies. BMJ. 2021 Jan 18;372:n120. PubMed: https://pubmed.gov/33461962. Full text: https://doi.org/10.1136/bmj.n120
Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021 Apr 15;6(58):eabi6950. PubMed: https://pubmed.gov/33858945. Full-text: https://doi.org/10.1126/sciimmunol.abi6950
Gorman MJ, Patel N, Guebre-Xabier M, et al. Collaboration between the Fab and Fc contribute to maximal protection against SARS-CoV-2 in nonhuman primates following NVX-CoV2373 subunit vaccine with Matrix-M™ vaccination. bioRxiv. 2021 Feb 5:2021.02.05.429759. PubMed: https://pubmed.gov/33564763. Full-text: https://doi.org/10.1101/2021.02.05.429759
Goupil R, Benlarbi M, Beaubien-Souligny W, et al. Short-term antibody response afer 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ. 2021 May 12:cmaj.210673. PubMed: https://pubmed.gov/33980499. Full-text: https://doi.org/10.1503/cmaj.210673
GOV.UK 20201230. Regulatory approval of COVID-19 Vaccine AstraZeneca. https://www.gov.uk 2020, published 30 December. Full texts: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca
Greaney AJ, Loes AN, Crawford KHD, et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021 Mar 10;29(3):463-476.e6. PubMed: https://pubmed.gov/33592168. Full text: https://doi.org/10.1016/j.chom.2021.02.003
Greaney AJ, Starr TN, Gilchuk P, et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe. 2021 Jan 13;29(1):44-57.e9. PubMed: https://pubmed.gov/33259788. Full text: https://doi.org/10.1016/j.chom.2020.11.007
Greinacher A, Selleng K, Wesche J, et al. Towards Understanding ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). Reseach Square 2021, posted 20 April. Full text: https://www.researchsquare.com/article/rs-440461/v1
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021 Apr 9. PubMed: https://pubmed.gov/33835769. Full text: https://doi.org/10.1056/NEJMoa2104840
Greinacher A. Clinical Practice. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015 Jul 16;373(3):252-61. PubMed: https://pubmed.gov/26176382. Full text: https://doi.org/10.1056/NEJMcp1411910
Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020 May 20:S0092-8674(20)30610-3. PubMed: https://pubmed.gov/32473127. Full text: https://doi.org/10.1016/j.cell.2020.05.015
Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med 2020, published 1 September. Full text: https://doi.org/10.1056/NEJMoa2026116
Guebre-Xabier M, Patel N, Tian JH, et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine. 2020 Nov 25;38(50):7892-7896. PubMed: https://pubmed.gov/33139139. Full-text: https://doi.org/10.1016/j.vaccine.2020.10.064
Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet May 05, 2021. Full text: https://doi.org/10.1016/S0140-6736(21)00947-8
Haas EJ, McLaughlin JM, Khan F, et al. Infections, Hospitalizations, and Deaths Averted Via Direct Effects of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Vaccination Campaign, Israel. Lancet Preprint 2021b, posted 13 May. Full text: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3845367
Hacisuleyman E, Hale C, Saito Y, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med 2021, published 21 April. Full text: Full text: https://doi.org/10.1056/NEJMoa2105000
Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021 Apr 23:S0140-6736(21)00790-X. PubMed: https://pubmed.gov/33901423. Full-text: https://doi.org/10.1016/S0140-6736(21)00790-X
Halstead SB, Katzelnick L. COVID-19 Vaccines: Should We Fear ADE? J Infect Dis. 2020 Nov 13;222(12):1946-1950. PubMed: https://pubmed.gov/32785649. Full text: https://doi.org/10.1093/infdis/jiaa518
Hause AM, Gee J, Johnson T, et al. Anxiety-Related Adverse Event Clusters After Janssen COVID-19 Vaccination — Five U.S. Mass Vaccination Sites, April 2021. MMWR Morb Mortal Wkly Rep 2021;70:685–688. DOI: http://dx.doi.org/10.15585/mmwr.mm7018e3
Health Canada 20210505. Health Canada authorizes use of the Pfizer-BioNTech COVID-19 vaccine in children 12 to 15 years of age. Health Canada 2021, published 5 May. Full text: https://www.canada.ca/en/health-canada/news/2021/05/health-canada-authorizes-use-of-the-pfizer-biontech-covid-19-vaccine-in-children-12-to-15-years-of-age.html
Heaton PM. The Covid-19 Vaccine-Development Multiverse. N Engl J Med. 2020 Jul 14:NEJMe2025111. PubMed: https://pubmed.gov/32663910. Full text: https://doi.org/10.1056/NEJMe2025111
Hekele A, Bertholet S, Archer J, et al. Rapidly produced SAM((R)) vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2013 Aug;2(8):e52. PubMed: https://pubmed.gov/26038486. Full text: https://doi.org/10.1038/emi.2013.54
Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017 Feb 4;389(10068):505-518. PubMed: https://pubmed.gov/28017403. Full text: https://doi.org/10.1016/S0140-6736(16)32621-6
Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020 Nov 1;35(11):1973-1983. PubMed: https://pubmed.gov/33151337. Full-text: https://doi.org/10.1093/ndt/gfaa261
Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, et al. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P.1 variant transmission in Brazil: A test-negative case-control study. medRxiv 2021, posted 7 April. Full text: https://doi.org/10.1101/2021.04.07.21255081
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KWR, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2020, published 27 October. Full text: https://doi.org/10.1016/S1473-3099(20)30773-8
Hoffmann M, Hofmann-Winkler H, Krüger N, et al. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination. bioRxiv 2021, posted 5 May. Full text: https://doi.org/10.1101/2021.05.04.442663
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. PubMed: https://pubmed.gov/32142651. Full text: https://doi.org/10.1016/j.cell.2020.02.052
Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015 Mar;89(6):2995-3007. PubMed: https://pubmed.gov/25520500. Full text: https://doi.org/10.1128/JVI.02980-14
Houser KV, Broadbent AJ, Gretebeck L, et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017 Aug 17;13(8):e1006565. PubMed: https://pubmed.gov/28817732. Full text: https://doi.org/10.1371/journal.ppat.1006565
Huang B, Dai L, Wang H, et al. Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y.V2. Lancet Microbe. 2021 Apr 13. PubMed: https://pubmed.gov/33870240. Full-text: https://doi.org/10.1016/S2666-5247(21)00082-3
Hunter PR. Thrombosis and bleeding after the Oxford-AstraZeneca covid-19 vaccination. BMJ Opinion 2021, published 5 May. Full text: https://blogs.bmj.com/bmj/2021/05/05/thrombosis-and-bleeding-after-the-oxford-astrazeneca-covid-19-vaccination/
Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020 Sep 10;383(11):1085-1087. PubMed: https://pubmed.gov/32706954. Full text: https://doi.org/10.1056/NEJMc2025179
Ikegame S, Siddiquey M, Hung CT, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Res Sq. 2021 Apr 8:rs.3.rs-400230. PubMed: https://pubmed.gov/33851150. Full-text: https://doi.org/10.21203/rs.3.rs-400230/v1
Irani M, Siegal E, Jella A, Aster R, Padmanabhan A. Use of intravenous immunoglobulin G to treat spontaneous heparin-induced thrombocytopenia. Transfusion. 2019 Mar;59(3):931-934. PubMed: https://pubmed.gov/30556588. Full text: https://doi.org/10.1111/trf.15105
ISCIII 20210518. El uso combinado de las vacunas de AstraZeneca y Pfizer contra el SARS-CoV-2 ofrece una potente respuesta inmunitaria. Instituto de Salud Carlos III 2021, published 18 May. Full text: https://www.isciii.es/Noticias/Noticias/Paginas/Noticias/Presentaci%C3%B3n-resultados-preliminares-CombivacS.aspx
Iwata-Yoshikawa N, Uda A, Suzuki T, et al. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J Virol. 2014 Aug;88(15):8597-614. PubMed: https://pubmed.gov/24850731. Full text: https://doi.org/10.1128/JVI.00983-14
J&J 20210227. Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use – First Single-Shot Vaccine in Fight Against Global Pandemic. Johnson & Johnson 2021, published 27 February. Full text: https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic
J&J 20210312. Johnson & Johnson Single-Shot COVID-19 Vaccine Granted Emergency Use Listing by the World Health Organization. Johnson & Johnson 2021, published 12 March. Link: https://www.jnj.com/johnson-johnson-single-shot-covid-19-vaccine-granted-emergency-use-listing-by-the-world-health-organization
Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020 Jul 14:NEJMoa2022483. PubMed: https://pubmed.gov/32663912. Full text: https://doi.org/10.1056/NEJMoa2022483
Jamrozik E, Selgelid MJ. COVID-19 human challenge studies: ethical issues. Lancet Infect Dis. 2020 May 29:S1473-3099(20)30438-2. PubMed: https://pubmed.gov/32479747. Full text: https://doi.org/10.1016/S1473-3099(20)30438-2
JCVI 20201230. JCVI issues advice on the AstraZeneca COVID-19 vaccine. Joint Committee on Vaccination and Immunisation (JCVI) 2020, published 30 December 2020. Full text: https://www.gov.uk/government/news/jcvi-issues-advice-on-the-astrazeneca-covid-19-vaccine
JCVI 20210407. Statement from the Joint Committee on Vaccination and Immunisation (JCVI) on the use of the AstraZeneca COVID-19 vaccine. Joint Committee on Vaccination and Immunisation (JCVI) 2021, published 7 April. Full text: https://www.gov.uk/government/publications/use-of-the-astrazeneca-covid-19-vaccine-jcvi-statement
JCVI 20210507. JCVI advises on COVID-19 vaccine for people aged under 40. Joint Committee on Vaccination and Immunisation (JCVI) 2021, published 7 May. Full text: https://www.gov.uk/government/news/jcvi-advises-on-covid-19-vaccine-for-people-aged-under-40
Jeyanathan M, Afkhami S, Smaill F, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 2020, published 4 September. Full text: https://doi.org/10.1038/s41577-020-00434-6
JNJ 20210129. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. Johnson & Johnson 2021, published 29 January. Full text: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial
Johnston MS, Galan A, Watsky KL, Little AJ. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021 May 12. PubMed: https://pubmed.gov/33978670. Full-text: https://doi.org/10.1001/jamadermatol.2021.1214
Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet. 2021 Feb 20;397(10275):642-643. PubMed: https://pubmed.gov/33545098. Full-text: https://doi.org/10.1016/S0140-6736(21)00191-4
Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 Aug;584(7819):115-119. PubMed: https://pubmed.gov/32454513. Full text: https://doi.org/10.1038/s41586-020-2380-z
Juhl D, Eichler P, Lubenow N, Strobel U, Wessel A, Greinacher A. Incidence and clinical significance of anti-PF4/heparin antibodies of the IgG, IgM, and IgA class in 755 consecutive patient samples referred for diagnostic testing for heparin-induced thrombocytopenia. Eur J Haematol. 2006 May;76(5):420-6. PubMed: https://pubmed.gov/16466367. Full text: https://doi.org/10.1111/j.1600-0609.2005.00621.x
Kahn JP, Henry LM, Mastroianni C, et al. Opinion: For now, it’s unethical to use human challenge studies for SARS-CoV-2 vaccine development. PNAS October 29, 2020. Full text: https://doi.org/10.1073/pnas.2021189117
Kalimuddin S, Tham CY, Qui M, et al. Early T cell and binding antibody responses are associated with Covid-19 RNA vaccine efficacy onset. Med (N Y). 2021 Apr 8. PubMed: https://pubmed.gov/33851143. Full text: https://doi.org/10.1016/j.medj.2021.04.003
Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165-75. PubMed: https://pubmed.gov/16111635. Full text: https://doi.org/10.1016/j.immuni.2005.06.008
Karikó K, Muramatsu H, Keller JM, Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol Ther. 2012 May;20(5):948-53. PubMed: https://pubmed.gov/22334017. Full text: https://doi.org/10.1038/mt.2012.7
Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008 Nov;16(11):1833-40. PubMed: https://pubmed.gov/18797453. Full text: https://doi.org/10.1038/mt.2008.200
Karim SSA. Vaccines and SARS-CoV-2 variants: the urgent need for a correlate of protection. Lancet. 2021 Apr 3;397(10281):1263-1264. PubMed: https://pubmed.gov/33765410. Full text: https://doi.org/10.1016/S0140-6736(21)00468-2
Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med 2020; 383:2320-2332. Full text: https://doi.org/10.1056/NEJMoa2026920
Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422-34. PubMed: PubMed: https://pubmed.gov/4305198. Full text: https://doi.org/10.1093/oxfordjournals.aje.a120955
Kim YI, Kim SG, Kim SM, et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020 Apr 5. pii: S1931-3128(20)30187-6. PubMed: https://pubmed.gov/32259477. Full text: https://doi.org/10.1016/j.chom.2020.03.023
Kistler KE, Bedford T. Evidence for adaptive evolution in the receptor-binding domain of seasonal coronaviruses. bioRxiv 2020, posted 30 October. Full text: https://doi.org/10.1101/2020.10.30.352914
Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2020 Dec 8:S0140-6736(20)32623-4. PubMed: https://pubmed.gov/33306990. Full text: https://doi.org/10.1016/S0140-6736(20)32623-4
Krammer F, Srivastava K, Alshammary H, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021 Apr 8;384(14):1372-1374. PubMed: https://pubmed.gov/33691060. Full-text: https://doi.org/10.1056/NEJMc2101667
Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. PubMed: https://pubmed.gov/32967006. Full text: https://doi.org/10.1038/s41586-020-2798-3
Kremser P, Mann P, Bosch J, et al. Phase 1 Assessment of the Safety and Immunogenicity of an mRNA-Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. medRxiv 2020, posted 9 November. Full text: https://doi.org/10.1101/2020.11.09.20228551
Kupferschmidt 20200728. ‘Vaccine nationalism’ threatens global plan to distribute COVID-19 shots fairly. Science 2020, 28 July. Full text: https://www.sciencemag.org/news/2020/07/vaccine-nationalism-threatens-global-plan-distribute-covid-19-shots-fairly
Kupferschmidt 20201220. Mutant coronavirus in the United Kingdom sets off alarms, but its importance remains unclear. Nature 2020, published 20 December. Full text: https://www.sciencemag.org/news/2020/12/mutant-coronavirus-united-kingdom-sets-alarms-its-importance-remains-unclear
Kupferschmidt 20201223. U.K. variant puts spotlight on immunocompromised patients’ role in the COVID-19 pandemic. Nature 2020, published 23 December. Full text: https://www.sciencemag.org/news/2020/12/uk-variant-puts-spotlight-immunocompromised-patients-role-covid-19-pandemic
LaFraniere S, Thomas K, Weiland N, Gelles D, Stolberg SG, Grady D. Politics, Science and the Remarkable Race for a Coronavirus Vaccine. The New York Times 2020, published 21 November. Full text: https://www.nytimes.com/2020/11/21/us/politics/coronavirus-vaccine.html
Lambert PH, Ambrosino DM, Andersen SR, et al. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: Assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020 Jun 26;38(31):4783-4791. PubMed: https://pubmed.gov/32507409. Full text: https://doi.org/10.1016/j.vaccine.2020.05.064
Lancet 20201121. COVID-19 vaccines: no time for complacency. Lancet 2020, published 21 November. Full text: https://doi.org/10.1016/S0140-6736(20)32472-7
Lancet Microbe 20201218. COVID-19 vaccines: the pandemic will not end overnight. The Lancet Microbe December 18, 2020. Full text: https://doi.org/10.1016/S2666-5247(20)30226-3
Lauring AS, Hodcroft EB. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA 2021, published 6 January. Full text: https://doi.org/10.1001/jama.2020.27124
Ledford H. Antibody therapies could be a bridge to a coronavirus vaccine — but will the world benefit? Nature 2020, published 11 August. Full text: https://www.nature.com/articles/d41586-020-02360-y
Ledford H. The race to make COVID antibody therapies cheaper and more potent. Nature 2020b, published 23 October. Full text: https://www.nature.com/articles/d41586-020-02965-3
Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021 May 1;96(5):534-537. PubMed: https://pubmed.gov/33606296. Full text: https://doi.org/10.1002/ajh.26132
Leung K, Shum MH, Leung GM, Lam TT, Wu JT. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021 Jan;26(1). PubMed: https://pubmed.gov/33413740. Full text: https://doi.org/10.2807/1560-7917.ES.2020.26.1.2002106
Levi R, Azzolini E, Pozzi C, et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in recovered individuals with symptomatic COVID-19. J Clin Invest. 2021 May 6:149154. PubMed: https://pubmed.gov/33956667. Full-text: https://doi.org/10.1172/JCI149154
Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020 Dec;35(12):1123-1138. PubMed: https://pubmed.gov/33289900. Full text: https://doi.org/10.1007/s10654-020-00698-1
Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008 Oct 15;181(8):5490-500. PubMed: https://pubmed.gov/18832706. Full text: https://doi.org/10.4049/jimmunol.181.8.5490
Libster R, Marc PG, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med 2021, published 6 January. Full text: https://doi.org/10.1056/NEJMoa2033700
Lipsitch M, Dean NE. Understanding COVID-19 vaccine efficacy. Science. 2020 Oct 21:eabe5938. PubMed: https://pubmed.gov/33087460. Full text: https://doi.org/10.1126/science.abe5938
Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019 Feb 21;4(4). pii: 123158. PubMed: https://pubmed.gov/30830861. Full text: https://doi.org/123158
Liu Y, Liu J, Xia H, et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N Engl J Med. 2021b May 12. PubMed: https://pubmed.gov/33979486. Full-text: https://doi.org/10.1056/NEJMc2106083
Liu Y, Liu J, Xia H, et al. Neutralizing Activity of BNT162b2-Elicited Serum – Preliminary Report. N Engl J Med. 2021 Feb 17. PubMed: https://pubmed.gov/33596352. Full text: https://doi.org/10.1056/NEJMc2102017
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 Feb 20;397(10275):671-681. PubMed: https://pubmed.gov/33545094. Full-text: https://doi.org/10.1016/S0140-6736(21)00234-8
Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020 Sep 26;396(10255):887-897. PubMed: https://pubmed.gov/32896291. Full-text: https://doi.org/10.1016/S0140-6736(20)31866-3
Lokugamage KG, Yoshikawa-Iwata N, Ito N, et al. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008 Feb 6;26(6):797-808. PubMed: https://pubmed.gov/18191004. Full text: https://doi.org/10.1016/j.vaccine.2007.11.092
Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 Aug;26(8):1200-1204. PubMed: https://pubmed.gov/32555424. Full text: https://doi.org/10.1038/s41591-020-0965-6
Low JS, Vaqueirinho D, Mele F, et al. Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2. Science 2021, published 18 May. Full text: https://doi.org/10.1126/science.abg8985
Lowe D. Brazil Rejects the Gamaleya Vaccine. Science 2021, published 28 April. Full text: https://blogs.sciencemag.org/pipeline/archives/2021/04/28/brazil-rejects-the-gamaleya-vaccine
Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009 Jun;21(3):346-51. PubMed: https://pubmed.gov/19500964. Full-text: https://doi.org/10.1016/j.coi.2009.05.016
Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020 May 21;382(21):1969-1973. PubMed: https://pubmed.gov/32227757. Full text: https://doi.org/10.1056/NEJMp2005630
Lustig Y, Nemet I, Kliker L, et al. Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2. N Engl J Med. 2021 Apr 7. PubMed: https://pubmed.gov/33826815. Full text: https://doi.org/10.1056/NEJMc2104036
Machemer T. A Brief History of Human Challenge Trials. Smithsonian Magazine 2020, published 16 December. Full text: https://www.smithsonianmag.com/science-nature/brief-history-human-challenge-trials-180976556/
Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 Mar 16. PubMed: https://pubmed.gov/33725432. Full text: https://doi.org/10.1056/NEJMoa2102214
Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015 Jan 1;211(1):80-90. PubMed: https://pubmed.gov/25030060. Full text: https://doi.org/10.1093/infdis/jiu396
Mallapaty 20210318. Has COVID peaked? Maybe, but it’s too soon to be sure. Nature. 2021 Mar;591(7851):512-513. PubMed: https://pubmed.gov/33737737. Full text: https://doi.org/10.1038/d41586-021-00705-9
Mallapaty 20210421. India’s massive COVID surge puzzles scientists. Nature. 2021 Apr 21. PubMed: https://pubmed.gov/33883710. Full text: https://doi.org/10.1038/d41586-021-01059-y
Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021 Mar 20;397(10279):1057-1058. PubMed: https://pubmed.gov/33640038. Full-text: https://doi.org/10.1016/S0140-6736(21)00501-8
Marston HD, Paules CI, Fauci AS. Monoclonal Antibodies for Emerging Infectious Diseases – Borrowing from History. N Engl J Med. 2018 Apr 19;378(16):1469-1472. PubMed: https://pubmed.gov/29513615. Full text: https://doi.org/10.1056/NEJMp1802256
Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020 Oct 2;370(6512):89-94. PubMed: https://pubmed.gov/32753554. Full text: https://doi.org/10.1126/science.abd3871
Mazzoni A, Di Lauria N, Maggi L, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in recovered COVID-19 subjects. J Clin Invest. 2021 May 3:149150. PubMed: https://pubmed.gov/33939647. Full-text: https://doi.org/10.1172/JCI149150
McPartlin SO, Morrison J, Rohrig A, Weijer C. Covid-19 vaccines: Should we allow human challenge studies to infect healthy volunteers with SARS-CoV-2? BMJ. 2020 Nov 9;371:m4258. PubMed: https://pubmed.gov/33168564. Full text: https://doi.org/10.1136/bmj.m4258
MHRA 20210401. Coronavirus vaccine – weekly summary of Yellow Card reporting. Medicines & Healthcare products Regulatory Agency (UK). Published 1 April, accessed 2 April. Full text: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
MHRA 20210422. Coronavirus vaccine – weekly summary of Yellow Card reporting. Medicines & Healthcare products Regulatory Agency (MHRA) 2021, updated 22 April (accessed 23 April). Full text: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
Moderna 20201210. Moderna Announces First Participants Dosed in Phase 2/3 Study of COVID-19 Vaccine Candidate in Adolescents. Moderna 2020, published 10 December. Full text: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participants-dosed-phase-23-study-covid
Moderna 20210315. First Participants Dosed in Phase 1 Study Evaluating mRNA-1283, Moderna’s Next Generation COVID-19 Vaccine. Moderna 2020, published 10 December. Full text: https://investors.modernatx.com/news-releases/news-release-details/first-participants-dosed-phase-1-study-evaluating-mrna-1283
Moderna 20210316. Moderna Announces First Participants Dosed in Phase 2/3 Study of COVID-19 Vaccine Candidate in Pediatric Population. Moderna 2020, published 10 December. Full text: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participants-dosed-phase-23-study-0
Moderna 20210413. Moderna Provides Clinical and Supply Updates on COVID-19 Vaccine Program Ahead of 2nd Annual Vaccines Day. Moderna 2021, published 13 April. Full text: https://investors.modernatx.com/news-releases/news-release-details/moderna-provides-clinical-and-supply-updates-covid-19-vaccine
Moderna 20210505. Moderna Announces Positive Initial Booster Data Against SARS-CoV-2 Variants of Concern. Moderna 2021, published 5 May. Full text: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-initial-booster-data-against-sars-cov
Mohandas S, Yadav PD, Shete-Aich A, et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS- CoV-2 vaccine candidates in the Syrian hamster model. iScience. 2021 Feb 19;24(2):102054. PubMed: https://pubmed.gov/33521604. Full-text: https://doi.org/10.1016/j.isci.2021.102054
Moyo-Gwete T, Madzivhandila M, Makhado Z, et al. Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351). N Engl J Med 2021, published 7 April. Full text: https://doi.org/10.1056/NEJMc2104192
Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021 Apr 14. PubMed: https://pubmed.gov/33852795. Full text: https://doi.org/10.1056/NEJMc2105869
Muller CP. Can integrated post-exposure vaccination against SARS-COV2 mitigate severe disease? Lancet Regional Health 2021, published 17 May. Full text: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(21)00095-8/fulltext
Mustafa SS, Ramsey A, Staicu ML. Administration of a Second Dose of the Moderna COVID-19 Vaccine After an Immediate Hypersensitivity Reaction With the First Dose: Two Case Reports. Ann Intern Med. 2021 Apr 6. PubMed: https://pubmed.gov/33819057. Full text: https://doi.org/10.7326/L21-0104
Nature 20210330. It’s time to consider a patent reprieve for COVID vaccines. Nature. 2021 Apr;592(7852):7. PubMed: https://pubmed.gov/33785920. Full text: https://doi.org/10.1038/d41586-021-00863-w
Neidleman J, Luo X, McGregor M, et al. mRNA vaccine-induced SARS-CoV-2-specific T cells recognize B.1.1.7 and B.1.351 variants but differ in longevity and homing properties depending on prior infection status. bioRxiv 2021, posted 12 May. Full text: https://doi.org/10.1101/2021.05.12.443888
Ng K, Faulkner N, Cornish G, et al. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. bioRxiv 2020, posted 15 May. Full text: https://doi.org/10.1101/2020.05.14.095414
Ngono AE, Shresta S. Immune Response to Dengue and Zika. Annu Rev Immunol. 2018 Apr 26;36:279-308. PubMed: https://pubmed.gov/29345964. Full text: https://doi.org/10.1146/annurev-immunol-042617-053142
Novavax 20210311. Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. Novavax 2021, 11 March press release. Full-text: https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0
Novavax 20210311. Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. Novavax 2021, 11 March press release. Full text: https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0
Novavax 20210506. Novavax and Gavi Execute Advance Purchase Agreement for COVID-19 Vaccine for COVAX Facility. Novavax 2021, 6 May press release. Full text: https://ir.novavax.com/news-releases/news-release-details/novavax-and-gavi-execute-advance-purchase-agreement-covid-19
NYTimes 20210414. The E.U.’s vaccination campaign gets a lift from Pfizer as it promises a big switch in strategy. The New York Times 2021, published 14 April. Full text: https://www.nytimes.com/2021/04/14/world/pfizer-europe-coronavirus-vaccine.html
Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie. 2021 Apr 1. PubMed: https://pubmed.gov/33822348. Full text: https://doi.org/10.1055/a-1469-7481
Oliver S, Gargano J, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. ePub: 13 December 2020. Full text: http://dx.doi.org/10.15585/mmwr.mm6950e2
Oliver S, Gargano J, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1653-1656. Full text: http://dx.doi.org/10.15585/mmwr.mm695152e1
Openshaw PJ, Culley FJ, Olszewska W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine. 2001 Oct 15;20 Suppl 1:S27-31. PubMed: https://pubmed.gov/11587806. Full text: https://doi.org/10.1016/s0264-410x(01)00301-2
Oxford University 20210207. ChAdOx1 nCov-19 provides minimal protection against mild-moderate COVID-19 infection from B.1.351 coronavirus variant in young South African adults. Oxford University News 2021, published 7 February. Full text: https://www.ox.ac.uk/news/2021-02-07-chadox1-ncov-19-provides-minimal-protection-against-mild-moderate-covid-19-infection
Oxford University 20210212. Oxford University extends COVID-19 vaccine study to children. Oxford University News 2021, published 12 February. Full text: https://www.ox.ac.uk/news/2021-02-12-oxford-university-extends-covid-19-vaccine-study-children
Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen-specific CD4+ T cells guides coordinated humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination. bioRxiv 2021, posted 22 April. Full text: https://doi.org/10.1101/2021.04.21.440862
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. PubMed: https://pubmed.gov/29326426. Full-text: https://doi.org/10.1038/nrd.2017.243
Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015 Nov 10;217:345-51. PubMed: https://pubmed.gov/26264835. Full text: https://doi.org/10.1016/j.jconrel.2015.08.007
Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people [preprint]. National Infection Service & University of Birmingham; Received 13 May 2021. Press release: https://www.birmingham.ac.uk/news/latest/2021/05/covid-pfizer-vaccination-interval-antibody-response.aspx + https://www.uk-cic.org/news/delaying-second-pfizer-vaccines-12-weeks-significantly-increases-antibody-responses-older
Pathak EB. Convalescent plasma is ineffective for covid-19. BMJ. 2020 Oct 22;371:m4072. PubMed: https://pubmed.gov/33093025. Full text: https://doi.org/10.1136/bmj.m4072
Pezzotti P, Fabiani M, Urdiales AM, et al. Impatto della vaccinazione COVID-19 sul rischio di infezione da SARS-CoV-2 e successivo ricovero e decesso in Italia (27.12.2020 – 03.05.2021). Istituto Superiore di Sanità (Italia) 2021, published 15 May. Full text: https://www.epicentro.iss.it/vaccini/covid-19-report-valutazione-vaccinazione
Pfizer 20210225. Pfizer and BioNTech Initiate a Study as Part of Broad Development Plan to Evaluate COVID-19 Booster and New Vaccine Variants. Press release, 25 February. Full text: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-part-broad-development
Pfizer 20210331. Pfizer-BioNTech Announce Positive Topline Results of Pivotal COVID-19 Vaccine Study in Adolescents. Pfizer press release 2021, published 31 March. Full text: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal
Pfizer-BioNTech 20210331. Pfizer-BioNTech Announce Positive Topline Results of Pivotal COVID-19 Vaccine Study in Adolescents. Press release, 31 March 2021. Full text: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal
Pilishvili T, Fleming-Dutra KE, Farrar JL, et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel — 33 U.S. Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 14 May 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7020e2
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 10:NEJMoa2034577. PubMed: https://pubmed.gov/33301246. Full text: https://doi.org/10.1056/NEJMoa2034577
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol (2020). Full text: https://doi.org/10.1038/s41577-020-00479-7
Posten D, Weisblum Y, Wise H, et al. Absence of SARS-CoV-2 neutralizing activity in pre-pandemic sera from individuals with recent seasonal coronavirus infection. medRxiv 2020, published 11 October. Full text: https://doi.org/10.1101/2020.10.08.20209650
Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021 May 5;373:n1114. PubMed: https://pubmed.gov/33952445. Full-text: https://doi.org/10.1136/bmj.n1114
Pritchard E, Matthews P, Stoesser N, et al. Impact of vaccination on SARS-CoV-2 cases in the community: a population-based study using the UK COVID-19 Infection Survey. medRxiv 2021, posted 23 April. Full text: https://doi.org/10.1101/2021.04.22.21255913
Public Health England 20210108. Investigation of novel SARS-COV-2 variant: Variant of Concern 202012/01. UK Government 2021, updated 8 January. Full text: https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201 – accessed 12 January 2021.
Public Health England 20210222. PHE monitoring of the early impact and effectiveness of COVID-19 vaccination in England. UK Government 2021, 22 February; accessed 5 March 2021. Full text: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963532/COVID-19_vaccine_effectiveness_surveillance_report_February_2021_FINAL.pdf
Public Health England 20210507. SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 10. UK Government 2021, updated 7 May. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf – accessed 11 May 2021.
Public Health England 20210513. SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 11. UK Government 2021, updated 13 May. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986380/Variants_of_Concern_VOC_Technical_Briefing_11_England.pdf – accessed 15 May 2021.
Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021 May 1;397(10285):1658-1667. PubMed: https://pubmed.gov/33915094. Full-text: https://doi.org/10.1016/S0140-6736(21)00682-6
Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021 Dec 19;396(10267):1979-1993. PubMed: https://pubmed.gov/33220855. Full text: https://doi.org/10.1016/S0140-6736(20)32466-1
Rauch S, Gooch K, Hall Y, et al. mRNA vaccine CVnCoV protects non-human primates from SARS-CoV-2 challenge infection. bioRxiv 2020, posted 23 December. Full text: https://doi.org/10.1101/2020.12.23.424138
Rauch S, Roth N, Schwendt K, Fotin-Mleczek M, Mueller SO, Petsch B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines. 2021 Apr 16;6(1):57. PubMed: https://pubmed.gov/33863911. Full-text: https://doi.org/10.1038/s41541-021-00311-w
Regeneron. REGN-COV-2 Antibody Cocktail Program Updates, September 29, 2020. https://investor.regeneron.com/static-files/a596a85e-e72d-4529-8eb5-d52d87a99070
Reuters 20200702. Tesla to make molecule printers for German COVID-19 vaccine developer CureVac. Reuters 2020, published 2 July. Full text: https://www.reuters.com/article/us-health-coronavirus-tesla/tesla-to-make-molecule-printers-for-german-covid-19-vaccine-developer-curevac-idUSKBN243168
Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021 Apr 30:eabh1282. PubMed: https://pubmed.gov/33931567. Full-text: https://doi.org/10.1126/science.abh1282
Risma KA, Edwards KM, Hummell DS, et al. Potential Mechanisms of Anaphylaxis to COVID-19 mRNA Vaccines. J Allergy Clin Immunol. 2021 Apr 12:S0091-6749(21)00565-0. PubMed: https://pubmed.gov/33857566. Full text: https://doi.org/10.1016/j.jaci.2021.04.002
Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 Aug;584(7821):437-442. PubMed: https://pubmed.gov/32555388. Full text: https://doi.org/10.1038/s41586-020-2456-9
Rossman H, Shilo S, Meir T, Gorfine L, Shalit U, Segal E. COVID-19 dynamics after a national immunization program in Israel. Nat Med (2021). Full text: https://doi.org/10.1038/s41591-021-01337-2
Roth N, Schön J, Hoffmann D, et al. CV2CoV, an enhanced mRNA-based SARS-CoV-2 vaccine candidate, supports higher protein expression and improved immunogenicity in rats. bioRxiv 2021, posted 13 May. Full text: https://doi.org/10.1101/2021.05.13.443734
Rubin EJ, Baden LR, Morrissey S. Audio Interview: Vaccine Successes and Vaccine Adverse Events. N Engl J Med. 2021 Apr 15;384(15):e70. PubMed: https://pubmed.gov/33852785. Full text: https://doi.org/10.1056/NEJMe2106379
Rubin EJ, Longo DL. SARS-CoV-2 Vaccination – An Ounce (Actually, Much Less) of Prevention. N Engl J Med. 2020 Dec 10:NEJMe2034717. PubMed: https://pubmed.gov/33301245. Full text: https://doi.org/10.1056/NEJMe2034717
Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021 Apr 13;325(14):1467-1469. PubMed: https://pubmed.gov/33646292. Full-text: https://doi.org/10.1001/jama.2021.3341
Sadoff J, Davis K, Douoguih M. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination – Response from the Manufacturer. N Engl J Med. 2021 Apr 16. PubMed: https://pubmed.gov/33861522. Full text: https://doi.org/10.1056/NEJMc2106075
Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021b, published 21 April. Full text: https://doi.org/10.1056/NEJMoa2101544
Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021 Jan 13:NEJMoa2034201. PubMed: https://pubmed.gov/33440088. Full text: https://doi.org/10.1056/NEJMoa2034201
Safi M. Oxygen runs low during India’s Covid crisis – photo essay. The Guardian 2021, published 23 April. Full text: https://www.theguardian.com/world/2021/apr/23/indias-covid-nightmare-photo-essay
Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv 2020, posted 11 December. Full text: https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1
Sanofi 20201211. Sanofi and GSK announce a delay in their adjuvanted recombinant protein-based COVID-19 vaccine program to improve immune response in the elderly. Sanofi 2020, published 11 December. Full text: https://www.sanofi.com/en/media-room/press-releases/2020/2020-12-11-07-00-00
Sanofi 20210715. Sanofi and GSK COVID-19 vaccine candidate demonstrates strong immune responses across all adult age groups in Phase 2 trial. Sanofi 2021, published 17 May. Full text: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-17-07-30-00-2230312
Sapkal GN, Yadav PD, Ella R, et al. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B 1.1.7 variant of SARS-CoV-2. J Travel Med. 2021 Mar 27:taab051. PubMed: https://pubmed.gov/33772577. Full-text: https://doi.org/10.1093/jtm/taab051
Saunders KO, Lee E, Parks R, et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021 May 10. PubMed: https://pubmed.gov/33971664. Full-text: https://doi.org/10.1038/s41586-021-03594-0
Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med 2021, published 9 April. Full text: Full text: https://doi.org/10.1056/NEJMoa2104882
Schwartz JL. Evaluating and Deploying Covid-19 Vaccines — The Importance of Transparency, Scientific Integrity, and Public Trust. N Engl J Med 2020; 383:1703-1705. Full text: https://doi.org/10.1056/NEJMp2026393
Scully M, Singh D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021 Apr 16. PubMed: https://pubmed.gov/33861525. Full text: https://doi.org/10.1056/NEJMoa2105385
Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020 Oct 1;183(1):158-168.e14. PubMed: https://pubmed.gov/32979941. Full text: https://doi.org/10.1016/j.cell.2020.08.017
Selleng S, Selleng K, Friesecke S, et al. Prevalence and clinical implications of anti-PF4/heparin antibodies in intensive care patients: a prospective observational study. J Thromb Thrombolysis. 2015 Jan;39(1):60-7. PubMed: https://pubmed.gov/25002339. Full text: https://doi.org/10.1007/s11239-014-1105-2
Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol (2020). Full text: https://doi.org/10.1038/s41564-020-00813-8
Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. The Lancet 2021, published 12 May. Full text: https://doi.org/10.1016/S0140-6736(21)01115-6
Shay DK, Gee J, Su JR, et al. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep 2021;70:680–684. DOI: http://dx.doi.org/10.15585/mmwr.mm7018e2
Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021b Apr 14;29(4):529-539.e3. PubMed: https://pubmed.gov/33705729. Full-text: https://doi.org/10.1016/j.chom.2021.03.002
Shen X, Tang H, Pajon R, et al. Neutralization of SARS-CoV-2 Variants B.1.429 and B.1.351. NEJM April 7, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2103740
Shen X, Tang H, Pajon R, et al. Neutralization of SARS-CoV-2 Variants B.1.429 and B.1.351. N Engl J Med. 2021 Apr 7. PubMed: https://pubmed.gov/33826819. Full text: https://doi.org/10.1056/NEJMc2103740
Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021 Feb 12. PubMed: https://pubmed.gov/33576785. Full text: https://doi.org/10.1001/jama.2021.1967
Shimabukuro TT, Kim SY, Meyers TR, et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med 2021b, published 21 April. Full text: https://doi.org/10.1056/NEJMoa2104983
Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 May 5. PubMed: https://pubmed.gov/33951374. Full-text: https://doi.org/10.1056/NEJMoa2103055
Slaoui M, Hepburn M. Developing Safe and Effective Covid Vaccines — Operation Warp Speed’s Strategy and Approach. N Engl J Med 2020, published 26 August. Full text: https://doi.org/10.1056/NEJMp2027405
Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002 Sep 25;288(12):1471-2. PubMed: https://pubmed.gov/12243633. Full text: https://doi.org/10.1001/jama.288.12.1471-a
Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA. 2021 Mar 11:e213645. PubMed: https://pubmed.gov/33704352. Full text: https://doi.org/10.1001/jama.2021.3645
Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA March 11, 2021. https://jamanetwork.com/journals/jama/fullarticle/2777598
Taji L, Thomas D, Oliver MJ, et al. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ. 2021 Feb 22;193(8):E278-E284. PubMed: https://pubmed.gov/33542093. Full-text: https://doi.org/10.1503/cmaj.202601
Tang F, Quan Y, Xin ZT, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011 Jun 15;186(12):7264-8. PubMed: https://pubmed.gov/21576510. Full text: https://doi.org/10.4049/jimmunol.0903490
Tang J. What we know about the Indian Covid variant so far. The Guardian 2021, published 14 May. Full text: https://www.theguardian.com/commentisfree/2021/may/14/indian-covid-variant-vaccines-strain-data
Tegally H, Wilkonson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv 2020, posted 22 December. Full text: https://doi.org/10.1101/2020.12.21.20248640
Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years – United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021 May 7;70(18):674-679. PubMed: https://pubmed.gov/33956782. Full-text: https://doi.org/10.15585/mmwr.mm7018e1
Teran RA, Walblay KA, Shane EL, et al. Postvaccination SARS-CoV-2 Infections Among Skilled Nursing Facility Residents and Staff Members — Chicago, Illinois, December 2020–March 2021. MMWR 2021, published 21 April. Full text: https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e1.htm
Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021 May 14:blood.2021012217. PubMed: https://pubmed.gov/33988688. Full-text: https://doi.org/10.1182/blood.2021012217
Thomas K. Novavax Reports More Delays for Its Covid-19 Vaccine. The New York Times 2021, published 11 May. Full text: https://www.nytimes.com/2021/05/11/health/covid-vaccine-novavax-delays.html
Thompson MG, Burgess JL, Naleway AL, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. ePub: 29 March 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7013e3.htm
Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021 Jan 14;12(1):372. PubMed: https://pubmed.gov/33446655. Full-text: https://doi.org/10.1038/s41467-020-20653-8
To KK, Hung IF, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 Aug 25:ciaa1275. PubMed: https://pubmed.gov/32840608. Full text: https://doi.org/10.1093/cid/ciaa1275
Tonn T, Corman VM, Johnsen M, et al. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe 2020. Full text: https://doi.org/10.1016/S2666-5247(20)30037-9
Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in PEG allergic patients. J Allergy Clin Immunol. 2021 May 12:S0091-6749(21)00731-4. PubMed: https://pubmed.gov/33991580. Full-text: https://doi.org/10.1016/j.jaci.2021.04.032
Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. PubMed: https://pubmed.gov/22536382. Full text: https://doi.org/10.1371/journal.pone.0035421
Tu W, Gierada DS, Joe BN. COVID-19 Vaccination-Related Lymphadenopathy: What To Be Aware Of. Radiol Imaging Cancer. 2021 May;3(3):e210038. PubMed: https://pubmed.gov/33874733. Full-text: https://doi.org/10.1148/rycan.2021210038
Tufekci Z. The Mutated Virus Is a Ticking Time Bomb. The Atlantic 2020, published 31 December. Full text: https://www.theatlantic.com/science/archive/2020/12/virus-mutation-catastrophe/617531
Usher DA. South Africa and India push for COVID-19 patents ban. Lancet 2020, published 5 December. Full text: https://doi.org/10.1016/S0140-6736(20)32581-2
Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020. Full text: https://doi.org/10.1016/j.immuni.2020.05.002
Vaidyanathan G. Coronavirus variants are spreading in India — what scientists know so far. Nature 2021, published 11 May. Full text: https://www.nature.com/articles/d41586-021-01274-7
van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020 Jul 30. PubMed: https://pubmed.gov/32731258. Full text: https://doi.org/10.1038/s41586-020-2608-y
Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021 Apr 23;397(10285):1646-57. PubMed: https://pubmed.gov/33901420. Full-text: https://doi.org/10.1016/S0140-6736(21)00677-2
Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N Engl J Med 2021, published 19 May. Full text: https://doi.org/ 10.1056/NEJMc2106383
Vergara E. Big Chile study finds Chinese vaccine slashes COVID deaths. ABC News 2021, published 16 April. Full text: https://abcnews.go.com/Health/wireStory/big-chile-study-finds-chinese-vaccine-slashes-covid-77120616
Villa L, Krüger T, Seikrit C, et al. Time on previous renal replacement therapy is associated with worse outcomes of COVID-19 in a regional cohort of kidney transplant and dialysis patients. Medicine (Baltimore). 2021 Mar 12;100(10):e24893. PubMed: https://pubmed.gov/33725847. Full-text: https://doi.org/10.1097/MD.0000000000024893
Voloch CM, da Silva R, de Almeida LGP, et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. medRxiv 2020, posted 26 December. Full text: https://doi.org/10.1101/2020.12.23.20248598
Volz E, Mishra W, Chand M, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv 2021, posted 4 January. Full text: https://doi.org/10.1101/2020.12.30.20249034
Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99-111. PubMed: https://pubmed.gov/33306989. Full text: https://doi.org/10.1016/S0140-6736(20)32661-1
Wadman M. Fever, aches from Pfizer, Moderna jabs aren’t dangerous but may be intense for some. Science 2020, published 18 November. Full text: https://www.sciencemag.org/news/2020/11/fever-aches-pfizer-moderna-jabs-aren-t-dangerous-may-be-intense-some
Wadman M. The long shot. Science. 2020 Nov 6;370(6517):649-653. PubMed: https://pubmed.gov/33154120. Full-text: https://doi.org/10.1126/science.370.6517.649
Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, published 28 October. Full text: https://doi.org/10.1126/science.abd7728
Walker LM, Burton DR. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat Rev Immunol. 2018 May;18(5):297-308. PubMed: https://pubmed.gov/29379211. Full text: https://doi.org/10.1038/nri.2017.148
Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years — United States, May 2021. MMWR Morb Mortal Wkly Rep. ePub: 14 May 2021. Full text: http://dx.doi.org/10.15585/mmwr.mm7020e1
Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020 Dec 17;383(25):2439-2450. PubMed: https://pubmed.gov/33053279. Full text: https://doi.org/10.1056/NEJMoa2027906
Wang GL, Wang ZY, Duan LJ, et al. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N Engl J Med. 2021 Apr 6. PubMed: https://pubmed.gov/33822491. Full-text: https://doi.org/10.1056/NEJMc2103022
Wang H, Zhang Y, Huang B, et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020 Aug 6;182(3):713-721.e9. PubMed: https://pubmed.gov/32778225. Full text: https://doi.org/10.1016/j.cell.2020.06.008
Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 Mar 8. PubMed: https://pubmed.gov/33684923. Full-text: https://doi.org/10.1038/s41586-021-03398-2
Wang P, Wang M, Yu J, et al. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. bioRxiv 2021, posted 2 March. Full text: https://doi.org/10.1101/2021.03.01.433466
Wang Q, Zhang L, Kuwahara K, et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect Dis. 2016 May 13;2(5):361-76. PubMed: https://pubmed.gov/27627203. Full text: https://doi.org/10.1021/acsinfecdis.6b00006
Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis 2020, published 4 June. Full text: https://doi.org/10.1093/cid/ciaa721
Ward BJ, Gobeil P, Séguin A, et al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants. medRxiv 2020, posted 6 November. Full text: https://doi.org/10.1101/2020.11.04.20226282
Warkentin TE. Clinical picture of heparin-induced thrombocytopenia (HIT) and its differentiation from non-HIT thrombocytopenia. Thromb Haemost. 2016 Oct 28;116(5):813-822. PubMed: https://pubmed.gov/27656712. Full text: https://doi.org/10.1160/TH16-06-0435
WCC 20210407. Communicating the potential benefits and harms of the Astra-Zeneca COVID-19 vaccine. Winton Centre Cambridge 2021, published 7 April. Full text: https://wintoncentre.maths.cam.ac.uk/news/communicating-potential-benefits-and-harms-astra-zeneca-covid-19-vaccine/
Wec AZ, Wrapp D, Herbert AS, et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020 Aug 7;369(6504):731-736. PubMed: https://pubmed.gov/32540900. Full text: https://doi.org/10.1126/science.abc7424
Wee SL, Londoño E. A Second Chinese Coronavirus Vaccine Is Said to Be Effective. The New York Times 2021, published 7 January. Full text: https://www.nytimes.com/2021/01/07/business/china-coronavirus-vaccine-sinovac.html
Wee SL, Qin A. China Approves Covid-19 Vaccine as It Moves to Inoculate Millions. The New York Time 2020, published 30 December. Full text: https://www.nytimes.com/2020/12/30/business/china-vaccine.html
Weingartl H, Czub M, Czub S, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004 Nov;78(22):12672-6. PubMed: https://pubmed.gov/15507655. Full text: https://doi.org/10.1128/JVI.78.22.12672-12676.2004
Weis S, Scherag A, Baier M, et al. Seroprevalence of SARS-CoV-2 antibodies in an entirely PCR-sampled and quarantined community after a COVID-19 outbreak – the CoNAN study. medRxiv 2020, posted 17 July. Full text: https://doi.org/10.1101/2020.07.15.20154112
White EM, Yang X, Blackman C, Feifer RA, Gravenstein S, Mor V. Incident SARS-CoV-2 Infection among mRNA-Vaccinated and Unvaccinated Nursing Home Residents. N Engl J Med. 2021 May 19. PubMed: https://pubmed.gov/34010526. Full-text: https://doi.org/10.1056/NEJMc2104849
Wise 20210521. Covid-19: UK cases of variant from India rise by 160% in a week. BMJ. 2021 May 21;373:n1315. PubMed: https://pubmed.gov/34020965. Full-text: https://doi.org/10.1136/bmj.n1315
WHO 20200409. WHO target product profiles for COVID-19 vaccines. WHO 2020, published 9 April, accessed 2 September, 2020. Full text: https://www.who.int/who-documents-detail/who-target-product-profiles-for-covid-19-vaccines
WHO 20200506. Key criteria for the ethical acceptability of COVID-19 human challenge studies. WHO 2020, published 6 May. Full text: https://www.who.int/ethics/publications/key-criteria-ethical-acceptability-of-covid-19-human-challenge/en/
WHO 20201231. WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. WHO 2020, published 31 December. Full text: https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-use-validation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access
WHO 20210507. WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. WHO 2021, published 7 May. Full text: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation, Krause PR, Fleming TR, et al. Placebo-Controlled Trials of Covid-19 Vaccines – Why We Still Need Them. N Engl J Med. 2020 Dec 2. PubMed: https://pubmed.gov/33264543. Full text: https://doi.org/10.1056/NEJMp2033538
WHO Landscape. Draft landscape of COVID-19 candidate vaccines. Accessed 20 October 2020. Full text: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
Widge AT, Rouphael NG, Jackson LA, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2020 Dec 3:NEJMc2032195. PubMed: https://pubmed.gov/33270381. Full text: https://doi.org/10.1056/NEJMc2032195
Wong AHM, Tomlinson ACA, Zhou D, et al. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nat Commun. 2017 Nov 23;8(1):1735. PubMed: https://pubmed.gov/29170370. Full text: https://doi.org/10.1038/s41467-017-01706-x
Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 Mar 13;367(6483):1260-1263. PubMed: https://pubmed.gov/32075877. Full text: https://doi.org/10.1126/science.abb2507
Wu K, Choi A, Koch M, et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. medRxiv 2021, posted 6 May. Full text: https://www.medrxiv.org/content/10.1101/2021.05.05.21256716v1
Wu K, Choi A, Koch M, et al. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. medRxiv 2021, posted 13 April. Full text: https://doi.org/10.1101/2021.04.13.439482
Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020 Sep 8;324(10):951-960. PubMed: https://pubmed.gov/32789505. Full text: https://doi.org/10.1001/jama.2020.15543
Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021 Jan;21(1):39-51. PubMed: https://pubmed.gov/33069281. Full-text: https://doi.org/10.1016/S1473-3099(20)30831-8
Xie X, Zou J, Fonte-Garfias CR, et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv 2021, posted 7 January. Full text: https://doi.org/10.1101/2021.01.07.425740
Yadav PD, Ella R, Kumar S, et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat Commun. 2021 Mar 2;12(1):1386. PubMed: https://pubmed.gov/33654090. Full-text: https://doi.org/10.1038/s41467-021-21639-w
Yadav PD, Sapkal GN, Abraham P, et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. bioRxiv 2021, posted 23 April. Full text: https://doi.org/10.1101/2021.04.23.441101
Yasui F, Kai C, Kitabatake M, et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008 Nov 1;181(9):6337-48. PubMed: https://pubmed.gov/18941225. Full text: https://doi.org/10.4049/jimmunol.181.9.6337
Zahradnik J, Marciano S, Shemesh M, et al. SARS-CoV-2 RBD in vitro evolution follows contagious mutation spread, yet generates an able infection inhibitor. bioRxiv 2021, posted 8 January. Full text: https://www.biorxiv.org/content/10.1101/2021.01.06.425392v2
Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2020 Nov 17:S1473-3099(20)30843-4. PubMed: https://pubmed.gov/33217362. Full text: https://doi.org/10.1016/S1473-3099(20)30843-4
Zhao J, Alshukairi AN, Baharoon SA, et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017 Aug 4;2(14):eaan5393. PubMed: https://pubmed.gov/28778905. Full text: https://doi.org/10.1126/sciimmunol.aan5393
Zhao J, Zhao J, Mangalam AK, et al. Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity. 2016 Jun 21;44(6):1379-91. PubMed: https://pubmed.gov/27287409. Full text: https://doi.org/10.1016/j.immuni.2016.05.006
Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020b Aug 15;396(10249):479-488. PubMed: https://pubmed.gov/32702299. Full text: https://doi.org/10.1016/S0140-6736(20)31605-6
Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020a Jun 13;395(10240):1845-1854. PubMed: https://pubmed.gov/32450106. Full text: https://doi.org/10.1016/S0140-6736(20)31208-3
[1] The vast majority of mRNA vaccinees received two doses within the usual vaccination schedule (BioNTech/Pfizer: 21 + 4 days, Moderna: 28 + 2). Of those receiving the AstraZeneca vaccine none received the second dose.
[2] Serious adverse events are defined as requiring hospitalization, deemed life-threatening, or resulting in persistent or significant disability/incapacity, another medically important condition, or death. The terms serious and severe are NOT synonymous. The general term severe is often used to describe the intensity (severity) of a specific event; the event itself, however, may be of relatively minor medical significance (such as a Grade 3 headache). This is NOT the same as serious, which is based on patient/event outcome and is usually associated with events that pose a threat to a patient’s life or ability to function. A severe AE (Grade 3 or 4) is not necessarily serious.
[3] Cerebral sinus vein thromboses are a rare health condition (Capecchi 2018) with a published background incidence of 0.2 to 1.57 per 100,000 person-years.
[4] HIT is a progressive thrombotic condition which can cause venous and arterial thrombosis, typically during the second week after exposure to heparin, especially after cardiac and orthopedic procedures (Warkentin 2016).
[5] In a study by Krammer et al., a single dose of mRNA vaccine in people with a history of SARS-CoV-2 infection (n = 67) elicited post-vaccination antibody titers exceeded the median antibody titers measured in participants without pre-existing immunity after the second vaccine dose (n = 43) by more than a factor of 6 (Krammer 2021) (80% received the Pfizer vaccine and 20% the Moderna vaccine).
[6] Licensed protein-based vaccines include the hepatitis B vaccine licensed in 1986, a flu vaccine approved in 2013 and the human papillomavirus vaccine for the prevention of of cervical cancer.
[7] Among 2684 participants who were SARS-CoV-2-seronegative at baseline (94% of them were HIV-negative and 6% were HIV-positive), symptomatic COVID-19 was observed in 15 participants in the vaccine group and in 29 participants in the placebo group.
