16 November
Daniell H, Naur SK, Esmaeili N, et al. Debulking SARS-COV-2 in saliva using angiotensin converting enzyme 2 in the chewing gum to decrease oral virus transmission and infection. Mol Therapy November 10, 2021. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00579-7
Nice idea. Chewing gum with virus trapping proteins offers a general affordable strategy to protect patients from most oral virus reinfections through debulking or minimizing transmission to others.
14 November
Lebreil AL, Greux V, Glenet M, et al. Surfaces and Air contamination by SARS-CoV-2 using High-flow Nasal Oxygenation or Assisted Mechanical Ventilation System in ICU rooms of COVID-19 Patients. The Journal of Infectious Diseases November 12, 2021, jiab564, https://doi.org/10.1093/infdis/jiab564
In this study, viral RNA environmental contamination was found in 76% of 100 surfaces samples and in 30% of 40 air samples without any viable virus detection by cell culture assays. No significant differences of viral RNA levels on surfaces and in ambient air were observed between rooms of patients with assisted mechanical ventilation and those of patients with high-flow nasal cannula systems.
1 November
Dixon BC, Fischer RS, Zhao H, et al. Contact and SARS-CoV-2 Infections Among College Football Athletes in the Southeastern Conference During the COVID-19 Pandemic. JAMA Netw Open October 29, 2021;4(10):e2135566. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2785602?resultClick=1
Good news first: Low risk during the match. In this analysis of US college athletes playing regular season football games during the COVID-19 pandemic, no instances of in-game SARS-CoV-2 transmission was found. Despite 12% of athletes testing positive for SARS-CoV-2, only 18 had competed during the preceding 48 hours, and no downstream infections to members of the opposing team were seen.
Pauser J, Schwarz C, Morgan J, et al. SARS-CoV-2 transmission during an indoor professional sporting event. Sci Rep October 20, 11(1):20723. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8528911/
But please, Joshua, don’t play indoors (at least not basketball). In total, 69 people were present at the 2nd division match of the German professional club basketball (21 players and 48 staff). After the event, one player of team B reported that he developed non-specific symptoms of a respiratory tract infection. Results, despite “standard hygiene concepts”: at least 36 infections (three severe).
Bown JC, Moshe M, Blackwell A, et al. Inactivation of SARS-CoV-2 in chlorinated swimming pool water. Water Res October 15, 2021. https://pubmed.ncbi.nlm.nih.gov/34619607/
Swimming is ok. This study found that chlorinated water that adheres to UK swimming pool guidelines is sufficient in reducing SARS-CoV-2 infectious titer by at least 3 orders of magnitude.
29 October
Amato-Lourenço LF, de Souza Xavier Costa N, Dantas KC, et al. Quantification of airborne SARS-CoV-2 genomic particles in different hospital settings. Sci Rep October 27, 2021, 11, 21284 (2021). https://doi.org/10.1038/s41598-021-00761-1
Widespread presence of contaminated aerosols in different hospital units dedicated to or not dedicated to COVID-19 and in the autopsy room, all without negative pressure ventilation systems.
27 October
Lee BU. A high attack rate of 90% of SARS-CoV-2 Delta variant infections in crew personnel on a single navy ship. Journal of Travel Medicine October 20, 2021, taab168, 20 October 2021 https://academic.oup.com/jtm/advance-article/doi/10.1093/jtm/taab168/6404468?searchresult=1
An explosive outbreak on a Navy ship. Within 25 days, 272 sailors out of 301 (unvaccinated) sailors (90.4%) were infected with the Delta variant. 209 (77%) were symptomatic. All recovered. Join the Navy?
19 October
Riley J, Huntley JM, Miller JA, Slaichert ALB, Brown GD. Mask effectiveness for preventing secondary cases of COVID-19, Johnson County, Iowa, USA. Emerg Infect Dis 2022, October 12, 2021. https://wwwnc.cdc.gov/eid/article/28/1/21-1591_article
In September 2020, the Iowa Department of Public Health released guidance stating that persons exposed to someone with COVID-19 need not quarantine if the case-patient and the contact wore face masks at the time of exposure. Mask use by both parties reduced the secondary attack rate by half, from 25.6% to 12.5%.
18 October
Sendi P, Baldan R, Thierstein M, et al. A multidimensional cross-sectional analysis of COVID-19 seroprevalence among a police officer cohort: The PoliCOV-19 study. Open Forum Infectious Diseases 2021, published 17 October. Full text: https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofab524/6398672
In theory, protests and police fieldwork might provide a high exposure environment for SARS-CoV-2 infections. However, in this study from Bern, Switzerland, the seroprevalence of anti-SARS-CoV-2 antibodies of police officers was comparable to that reported in the general population. In Switzerland, the personal protective equipment of the police seems to have been effective.
16 October
de Gier B, Andeweg S, Backer JA, et al. Vaccine effectiveness against SARS‐CoV‐2 transmission to household contacts during dominance of Delta variant (B.1.617.2), August‐September 2021, the Netherlands. medRxiv 2021, posted 14 October. Full text: https://doi.org/10.1101/2021.10.14.21264959
The authors compared secondary attack rates among household members between vaccinated and unvaccinated index cases. They estimated the effectiveness of full vaccination to be 63%.
14 October
Sera F, Armstrong B, Abbott S, et al. A cross-sectional analysis of meteorological factors and SARS-CoV-2 transmission in 409 cities across 26 countries. Nat Commun 12, 5968 (2021). Full text: https://www.nature.com/articles/s41467-021-25914-8
The authors found little evidence of meteorological conditions having influenced the early stages of local COVID-19 epidemics. Non-pharmaceutical interventions, i.e., population behaviors and government interventions, are far more important drivers of SARS-CoV-2 transmission.
12 October
Emecen AN, Keskin S, Boncukcu Eren E, et al. Impact of social contacts on SARS-CoV-2 exposure among healthcare workers. Occupational Medicine 2021, published 11 October. Full text: https://doi.org/10.1093/occmed/kqab141
Interactions between healthcare workers (HCW) may pose a high risk for SARS-CoV-2 exposure. In this study from Turkey, 260 of 329 exposed clusters (79%) were HCW-to-HCW contact clusters. High-risk exposure was higher in the HCW-to-HCW contacts (44%), when compared to patient-to-HCW contacts (5%).
29 September
Hu X, Liu Z, Liang J, et al. Environmental contamination of a quarantine hotel via SARS-CoV-2 positive travellers. Journal of Travel Medicine September 2021; taab148, https://academic.oup.com/jtm/advance-article/doi/10.1093/jtm/taab148/6377253?searchresult=1
Report on the extent of environmental contamination of a quarantine hotel via two SARS-CoV-2 VOC-positive travelers. The bathroom had the highest rate for SARS-CoV-2 positive samples. Except for the TV remote control, samples from the two bedrooms were negative.

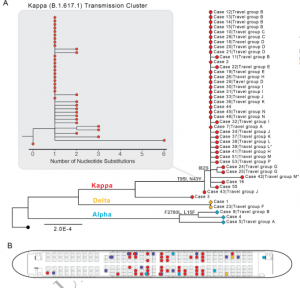
Timeline of the two travellers infected with two SARS-CoV-2 variants, distribution of environmental sampling sites in the quarantine room.
Benenson S, Ottolenghi M, Cohen MJ, et al. High attack rate of COVID-19 in an organized tour group of vaccinated travelers to Iceland. Journal of Travel Medicine, September 28, 2021, taab157, https://academic.oup.com/jtm/advance-article/doi/10.1093/jtm/taab157/6377252?searchresult=1
In a group of 25 twice-vaccinated Israeli travelers, 21 became infected (84%), despite negative pre-flight PCR tests, probably due to close and prolonged exposures during long bus drives.
28 September
Bigouette JP, Ford L, Segaloff HE, Langolf K, Kahrs J, Zochert T, et al. Association of shared living spaces and COVID-19 in university students, Wisconsin, USA, 2020. Emerg Infect Dis September 22, 2021. https://wwwnc.cdc.gov/eid/article/27/11/21-1000_article
Among 2187 students residing on a university campus, 528 (24%) received a COVID-19 diagnosis during the fall semester 2020. Not a big surprise: students sharing a bedroom or suite had approximately twice the odds of contracting COVID-19 as those living alone.
27 September
Lesieur E, Torrents J, Fina F, et al. Congenital infection of SARS-CoV-2 with intrauterine foetal death: a clinicopathological study with molecular analysis. Clinical Infectious Diseases September 23, 2021, ciab840, https://doi.org/10.1093/cid/ciab840
A case of in utero foetal death at 24+2 weeks of gestation that occurred seven days after the diagnosis of symptomatic SARS-CoV-2 infection in the mother.
23 September
Hu M, Wang J, Lin H, et al. Risk of SARS-CoV-2 Transmission among Air Passengers in China. Clinical Infectious Diseases September 21, 2021, ciab836, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab836/6373518
This analysis of 175 index cases among 5797 passengers on 177 airplanes came to the conclusion that the overall risk of SARS-CoV-2 transmission during domestic travel on planes is relatively low. However, the attack rates (AR) among travellers varied considerably. The seats immediately adjacent to the index cases had an AR of 9.2%, with a relative risk of 27.8 compared to other seats in the upper limit estimation. The middle seat had the highest AR.
Dhanasekaran V, Edwards KM, Xie R, et al. Air travel-related outbreak of multiple SARS-CoV-2 variants. Journal of Travel Medicine, September 21, 2021, taab149, https://academic.oup.com/jtm/advance-article/doi/10.1093/jtm/taab149/6372544?searchresult=1
And the thing is: sometimes it’s not only one single index case. In this report, a large cluster of 59 (!) cases were linked to a single flight with 146 passengers from New Delhi to Hong Kong in April 2021. Transmission of three VOI/VOC lineages occurred from at least seven index cases. Take your seats (or stay home).
SARS-CoV-2 transmission associated with a flight from New Delhi to Hong Kong in April 2021. Colors represent WHO variants and Pango lineage designations
16 September
Adenaiye OO, Lai J, Bueno de Mesquita J, et al. Infectious SARS-CoV-2 in Exhaled Aerosols and Efficacy of Masks During Early Mild Infection. Clinical Infectious Diseases September 14, 2021, ciab797, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab797/6370149
A heroic study and without a doubt the paper of the day. No one can say anymore that masks have not been studied: Oluwasanmi Adenaiye and colleagues recruited 49 COVID-19 cases to give blood, saliva, mid-turbinate and fomite (phone) swabs, and 30-minute (!) breath samples while vocalizing into a Gesundheit-II, with and without masks at up to two visits two days apart. Masks reduced viral RNA by 48% in fine aerosols and by 77% in coarse aerosols; cloth and surgical masks were not significantly different. The Alpha variant was associated with a 43-fold increase in fine aerosol viral RNA.
13 September
Rebmann T, Loux TM, Arnold LD, Charney R, Horton D, Gomel A. SARS-CoV-2 Transmission to Masked and Unmasked Close Contacts of University Students with COVID-19 — St. Louis, Missouri, January–May 2021. MMWR 2021;70:1245–1248. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7036a3.htm?s_cid=mm7036a3_w
An observational study in a university setting indicating that compared with only masked exposure, close contacts with any unmasked exposure had higher adjusted odds of a positive test result. Each additional exposure was associated with a 40% increase in the odds of a positive test.
12 September
Shah AS, Gribben C, Bishop J, et al. Effect of Vaccination on Transmission of SARS-CoV-2. NEJM September 8, 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2106757?query=featured_home
Incredible huge study on 194,362 household members of 144,525 health care workers from Scotland, among them 113,253 HCWs who had received at least one dose of either Pfizer–BioNTech or the AstraZeneca vaccine. The hazard ratio for a household member to become infected was 0.70 (95% CI, 0.63 to 0.78) for the period beginning 14 days after the first dose and 0.46 (95% CI, 0.30 to 0.70) for the period beginning 14 days after the second dose. As not all cases in the household members were transmitted from the health care worker, the effect of vaccination may be larger.
10 September
Dabisch PA, Biryukov J, Beck K. Seroconversion and fever are dose-dependent in a nonhuman primate model of inhalational COVID-19. PLOS Pathogens August 23, 2021. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009865
Small study in which 16 healthy, young adult macaques were exposed to small particle aerosols containing SARS-CoV-2, with calculated deposited doses. The probability of seroconversion and fever were both dose-dependent, but the median dose for seroconversion was significantly lower than that of fever. However, the study used an isolate from early in the pandemic (no variant).
9 September
Mishra A, Kumar N, Bhatia S, et al. SARS-CoV-2 Delta variant among Asiatic lions, India. Emerg Infect Dis 2021 August 31, 2021 https://wwwnc.cdc.gov/eid/article/27/10/21-1500_article
Identical SARS-CoV-2 infections in nine lions in a short period of time, indicating the possibility of lion-to-lion transmission.
8 September
Rosca EC, Heneghan C, Spencer EA, et al. Transmission of SARS-CoV-2 associated with aircraft travel: A systematic review. Journal of Travel Medicine September 3, 2021. taab133, https://doi.org/10.1093/jtm/taab133
Current evidence is very limited. Data suggests SARS-CoV-2 can be transmitted during aircraft travel, but published data do not permit any conclusive assessment of likelihood and/or extent.
3 September
Oster Y, Benenson S, Harpaz LY, et al. Association Between Exposure Characteristics and the Risk for COVID-19 Infection Among Health Care Workers With and Without BNT162b2 Vaccination. JAMA Netw Open September 1, 2021;4(9):e2125394. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2783675?resultClick=1
This case-control study found that exposure to SARS-CoV-2–positive household members was a risk factor associated with infection among vaccinated HCWs. Household exposure is usually longer and closer than casual exposure or exposure at work and does not include masking or distancing, exposing one to a higher infectious dose and thus being more contagious.
1 September
Madewell ZJ, Yang X, Longini Jr IM, et al. Factors Associated With Household Transmission of SARS-CoV-2An Updated Systematic Review and Meta-analysis. JAMA Netw Open August 27, 2021; 4(8):e2122240. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2783544?resultClick=1
In this updated systematic review and meta-analysis of 87 studies representing 1,249,163 household contacts from 30 countries, the estimated household secondary attack rate was 19%. The authors observed an increase in household transmission over time, perhaps owing to improved diagnostic procedures and tools, longer follow-up, more contagious variants, and different study locations.
28 August
Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science August 27, 2021. Vol. 373, Issue 6558, eabd9149. https://science.sciencemag.org/content/373/6558/eabd9149
Forget droplets and fomites. According to this comprehensive review, airborne transmission is the dominant form of transmission. The best arguments? The distinct difference between indoor and outdoor transmission (gravity-driven droplets behave identically indoors and outdoors) and the demonstrated role of poor ventilation (droplets and fomite transmission are not affected by ventilation).
27 August
Saretzki C, Bergmann O, Dahmann P, et al. Are small airplanes safe with regards to COVID-19 transmission? Journal of Travel Medicine August 20, 2021, taab105, https://doi.org/10.1093/jtm/taab105
Yes, they are – if the ventilation system is set on ‘high’. In this simulation study (an externally connected ventilation system was used to simulate the cockpit in-flight airflow for a four-seater general aviation aircraft) the airstream was marked with smoke for visualization.
25 August
Toumi A, Zhao H, Chhatwal J, et al. Association of Limited In-Person Attendance in US National Football League and National Collegiate Athletic Association Games With County-Level COVID-19 Cases. JAMA Netw Open August 17, 2021;4(8):e2119621. https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2783110
Are football games with limited in-person attendance associated with increased county-level COVID-19 cases? Probably not. This cross-sectional study of US counties that hosted National Football League games suggests that football games held with limited in-person attendance were not associated with increased COVID-19 cases in the counties where they were held.
24 August
Tinker SC, Szablewski CM, Litvintseva AP, Drenzek C, Voccio GE, Hunter MA, et al. Point-of-care antigen test for SARS-CoV-2 in asymptomatic college students. Emerg Infect Dis. 2021 Oct [date cited]. https://wwwnc.cdc.gov/eid/article/27/10/21-0080_article
Bad news for the BinaxNOW COVID-19 Ag Card. In this screening study of 1,540 asymptomatic college students, BinaxNOW missed many infections and showed only 20% overall sensitivity (among participants with culturable virus, sensitivity was 60%).
23 August
Smith JAE, Hopkins S, Turner C, et al. Public health impact of mass sporting and cultural events in a rising COVID-19 prevalence in England. Public Health England 2021, posted 21 August. Full text: https://khub.net/documents/135939561/338928724/Public+health+impact+of+mass+sporting+and+cultural+events+in+a+rising+COVID-19+prevalence+in+England.pdf/05204895-1576-1ee7-b41e-880d5d6b4f17
“The number of potentially infected persons attending Wembley stadium increased as the [EURO 2020] tournament progressed, reaching more than 2,000 at the EURO 2020 final despite event goers requiring a COVID pass for entry.” In the future, it might be “important to consider including mitigations for spectators to consider such as face coverings when travelling to and from events, minimising crowding in poorly ventilated indoors spaces such as bars and pubs where people may congregate to watch events. It is also important to minimise the risk of transmission from aerosol exposure related to singing and chanting in large groups by improving ventilation in enclosed spaces.” See also the Guardian article: Walker P. 9,000 Covid cases linked to Euro 2020 games in mass events scheme. The Guardian 2021, published 20 August. Full text: https://www.theguardian.com/world/2021/aug/20/9000-covid-cases-linked-to-euro-2020-games-in-mass-events-scheme
20 August
Branswell H. What’s safe to do during summer’s Covid surge? STAT asked public health experts about their own plans. Stat 2021, published 17 August. Full text: https://www.statnews.com/2021/08/17/whats-safe-to-do-during-summers-covid-surge-stat-asked-public-health-experts-about-their-own-plans/
Experts largely stay put.
Paul LA, Daneman N, Schwartz KL, et al. Association of Age and Pediatric Household Transmission of SARS-CoV-2 Infection. JAMA Pediatr. 2021 Aug 16. PubMed: https://pubmed.gov/34398179. Full text: https://doi.org/10.1001/jamapediatrics.2021.2770
In this Ontario study of 6280 households with pediatric index cases, 1717 households (27.3%) experienced secondary transmission.
“Younger children may have greater risk of transmitting SARS-CoV-2 to caregivers and siblings in the household than older children. In this cohort study of 6280 households with pediatric index cases, the adjusted odds of household transmission by children aged 0 to 3 years was 1.43 compared with children aged 14 to 17 years.”
Leibowitz AI, Siedner MJ, Tsai AC, Mohareb AM. Association Between Prison Crowding and COVID-19 Incidence Rates in Massachusetts Prisons, April 2020-January 2021. JAMA Intern Med. 2021 Aug 9:e214392. PubMed: https://pubmed.gov/34369964. Full text: https://doi.org/10.1001/jamainternmed.2021.4392
“In this study (…) including all incarcerated persons in 14 Massachusetts state prisons from April 2020 to January 2021 (…), COVID-19 incidence was significantly higher in prisons operating at a higher percentage of their design capacity and was significantly lower in prisons where a higher proportion of incarcerated people were housed in single-cell units.” See also the comment by Clemenzi-Allen AA, Pratt LA. Avoiding COVID-19 Outbreaks in Carceral Settings. JAMA Intern Med. 2021 Aug 9. PubMed: https://pubmed.gov/34369968. Full text: https://doi.org/10.1001/jamainternmed.2021.4389
18 August
Subbaraman N. How do vaccinated people spread Delta? What the science says. Nature 2021, published 12 August. Full text: https://www.nature.com/articles/d41586-021-02187-1
The Delta seems to be more likely than other variants to spread via vaccinated people. Although vaccinated people are probably infectious for a shorter period, they need to take precautions, especially in indoor settings. If you cannot avoid crowded indoor settings, see here: CDC 20210406. Improve How Your Mask Protects You. Centers for Disease Control 2021 (updated 6 April, accessed 15 August). Full text: https://www.cdc.gov/coronavirus/2019-ncov/your-health/effective-masks.html
17 August
Read JM, Green CA, Harrison EM, et al. Hospital-acquired SARS-CoV-2 infection in the UK’s first COVID-19 pandemic wave. Lancet 20212, published 12 August. Full text: https://doi.org/10.1016/S0140-6736(21)01786-4
Until the end of July 2020, 6.8% of patients with COVID-19 in 314 UK hospitals may have been infected after hospital admission, with a peak of 8.2% in mid-May. The authors conclusion, “As SARS-CoV-2 is likely to persist as an endemic or seasonal virus in coming years, it is critical to use the lessons learned so far in the pandemic to minimise the burden of hospital-acquired infections.”
8 August
Chen PZ, Koopmans M, Fisman DN, Gu FX. Understanding why superspreading drives the COVID-19 pandemic but not the H1N1 pandemic. Lancet Infect Dis 2021, published 2 August. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00406-0/fulltext
With SARS-CoV-2, fewer cases cause the majority of infections, and a greater proportion of infections tend to be linked to large clusters via superspreading events. Why is this phenomenon, called over-dispersion in transmissibility (k), a characteristic of the SARS-CoV-2 pandemic, not part of the 2009 influenza H1N1 pandemic? The authors discuss which virological factors mediate k.
3 August
Sikkens JJ, Buis DTP, Peters EJG, et al. Serologic Surveillance and Phylogenetic Analysis of SARS-CoV-2 Infection Among Hospital Health Care Workers. JAMA Netw Open. 2021 Jul 1;4(7):e2118554. PubMed: https://pubmed.gov/34319354. Full text: https://doi.org/10.1001/jamanetworkopen.2021.18554
In this cohort study of 801 hospital health care workers (HCWs), the risk of getting infected with SARS-CoV-2 was nearly 4-fold higher among HCWs on COVID-19 wards compared with HCWs not in patient care. There was no evidence for patient-to-HCW transmission but several occurrences of HCW-to-HCW transmission.
31 July
Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. ePub: 30 July 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7031e2
Bad news from Barnstable County, Massachusetts. In July 2021, following multiple summer events and large public gatherings, 469 COVID-19 cases were identified among Massachusetts residents who had traveled to the town during July 3–17. Intriguingly, 346 (74%) occurred in fully vaccinated persons. Even more intriguingly: cycle threshold values were similar among specimens from patients who were fully vaccinated and those who were not. The driving force behind the outbreak: the Delta variant.
29 July
Schrock JM, Ryan DT, Saber R, et al. Cohabitation With a Known Coronavirus Disease 2019 Case Is Associated With Greater Antibody Concentration and Symptom Severity in a Community-Based Sample of Seropositive Adults. Open Forum Infectious Diseases, Volume 8, Issue 7, 24 July 2021. Full text: https://doi.org/10.1093/ofid/ofab244
The authors find that those who lived with a known COVID-19 case exhibited greater symptom severity compared to those who did not live with a known COVID-19 case. Does the duration of viral exposure explain this relation between household exposure and symptom severity?
28 July
Else H, Baker N. Coronapod: the latest on COVID and sporting events. Nature 2021, published 24 July. Listen at https://www.nature.com/articles/d41586-021-02053-0
“Early in 2021 the United Kingdom, along with several other countries, allowed mass gatherings as part of a series of controlled studies aimed at better understanding the role events could play in the pandemic. The goal was to inform policy – however early results have provided limited data on viral transmission.” But, there were a lot of parties!
Bjorkman KK, Saldi TK, Lasda E, et al. Higher viral load drives infrequent SARS-CoV-2 transmission between asymptomatic residence hall roommates. J Infect Dis. 2021 Jul 24:jiab386. PubMed: https://pubmed.gov/34302469. Full-text: https://doi.org/10.1093/infdis/jiab386
Individuals who likely transmitted SARS-CoV-2 had an average viral load ~6.5-fold higher than individuals who did not (mean Cq 26.2 vs 28.9). Longitudinal data of 6408 students and 116 likely transmission events in which a second roommate tested positive within 14 days of the index roommate.
25 July
Else H. COVID and mass sport events: early studies yield limited insights. Nature 2021, published 22 July. Full text: https://www.nature.com/articles/d41586-021-02016-5
Is it safe to attend large gatherings? Maybe not yet.
23 July
Chu VT, Yousaf AR, Chang K, et al. Household Transmission of SARS-CoV-2 from Children and Adolescents. N Engl J Med. 2021 Jul 21. PubMed: https://pubmed.gov/34289272. Full-text: https://doi.org/10.1056/NEJMc2031915
SARS-CoV-2-infected children coming back from an overnight camp – transmission occurred in 18% of households (35/194); in these households, the secondary attack rate was 45%. The risk of household infection was lower among contacts who had practiced physical distancing (adjusted odds ratio, 0.4). Conclusion: “When feasible, children and adolescents with a known exposure to SARS-CoV-2 or a diagnosis of COVID-19 should remain at home and maintain physical distance from household members.”
21 July
Llibre JM, Videla S, Clotet B, Revollo B. Screening for SARS-CoV-2 Antigen Before a Live Indoor Music Concert: An Observational Study. Ann Intern Med 2021, published 20 July. Full text: https://doi.org/10.7326/M21-2278
Indoor mass-gathering events always have the potential to be super-spreading events. Here, the authors describe the potential of antigen-detecting rapid diagnostic tests (Ag-RDTs) to create safe environments (“same day rapid antigen tests, use of face masks, improved ventilation). More such experiences are needed to provide safe guidelines.
Coleman KK, Tay DJW, Tan KS, et al. Viral Load of SARS-CoV-2 in Respiratory Aerosols Emitted by COVID-19 Patients while Breathing, Talking, and Singing. medRxiv 2021, posted 19 July. Full text: https://doi.org/10.1101/2021.07.15.21260561
In this study, fine aerosols (≤ 5μm) produced by talking and singing contained more SARS-CoV-2 copies than coarse aerosols. “The largest proportion of SARS-CoV-2 RNA copies was emitted by singing (53%), followed by talking (41%) and breathing (6%).”
20 July
Li B, Deng A, Li K, et al. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant. medRxiv 2021, posted 12 July. Full-text: https://doi.org/10.1101/2021.07.07.21260122
Explaining the forces behind the Delta wave: 1) 1000 times higher viral load and 2) a reduced serial interval, 4 days instead of 6 (the serial interval is defined as the duration of time between a primary case patient having symptom onset and a secondary case patient having symptom onset).
Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics Facility – Oklahoma, April-May 2021. MMWR Morb Mortal Wkly Rep. 2021 Jul 16;70(28):1004-1007. PubMed: https://pubmed.gov/34264910. Full-text: https://doi.org/10.15585/mmwr.mm7028e2
In the coming months, avoid gyms. The Delta variant (B.1.617.2, first identified in India) is frighteningly transmissible (see article above) and will lead to increased attack rates, especially in households and in indoor sports settings. In this report, the attack rates at the gymnastics facility and in households were 20% and 53%, respectively.
5 July
Lindsley WG, Derk RC, Coyle JP, et al. Efficacy of Portable Air Cleaners and Masking for Reducing Indoor Exposure to Simulated Exhaled SARS-CoV-2 Aerosols — United States, 2021. MMWR Morb Mortal Wkly Rep. ePub: 2 July 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7027e1.htm?s_cid=mm7027e1_w
A simulated infected meeting participant who was exhaling aerosols was placed in a room with two simulated uninfected participants and a simulated uninfected speaker. Using two high efficiency particulate air (HEPA) cleaners close to the aerosol source reduced the aerosol exposure of the uninfected participants and speaker by up to 65%. A combination of HEPA air cleaners and universal masking reduced exposure by up to 90%.
22 June
Karan A, Klompas M, Tucker R, et al. Patients with Undiagnosed Covid-19 to Roommates in a Large Academic Medical Center. Clinical Infectious Diseases June 18, 2021, ciab564, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab564/6305137
Almost 40% of patients that shared a hospital room with someone with occult SARS-CoV-2 infection became infected. Among 31 exposed roommates, 12 tested positive within 14 days. The risk was highest for patients sharing rooms with individuals with very low Ct counts (< 22).
21 June
De Paula Eduardo F, Corrêa L, Heller D, et al. Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial. Heliyon June 17, 2021. https://doi.org/10.1016/j.heliyon.2021.e07346
Mouthwash with cetylpyridinium chloride + zinc and chlorhexidine gluconate resulted in significant reductions of the SARS-CoV-2 viral load in saliva up to 60 mins after rinsing, while hydrogen peroxide mouthwash resulted in a significant reduction up to 30 mins after rinsing.
20 June
Bi Q, Lessler J, Eckerle I, et al. Insights into household transmission of SARS-CoV-2 from a population-based serological survey. Nat Commun June 15, 2021, 12, 3643. https://doi.org/10.1038/s41467-021-23733-5
This was a cross-sectional, household-based population serosurvey of 4534 people ≥ 5 years from 2267 households enrolled April-June 2020 in Geneva, Switzerland. The risk of infection from exposure to a single infected household member aged ≥ 5 years (17.3%, 13.7-21.7) was more than three-times that of extra-household exposures over the first pandemic wave (5.1%, 4.5-5.8). Young children had a lower risk of infection from household members.
11 June
Razani N, Malekinejad M, Rutherford GW. Clarification regarding Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses, a Systematic Review. J Inf Dis June 4, 2021, jiab298, https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiab298/6291889
Update of a previous paper, supporting the initial conclusion that the risk of SARS-CoV-2 transmission is much lower outdoors than indoors. The proportion of infections happening outdoors is likely much lower than 10%, and most studies suggest that it is likely less than 1%.
7 June
Goyal A, Reeves DB, Thakkar N, et al. Slight reduction in SARS-CoV-2 exposure viral load due to masking results in a significant reduction in transmission with widespread implementation. Sci Rep 11, 11838 (2021). https://doi.org/10.1038/s41598-021-91338-5
This modeling study shows that the use of masks by both a potential transmitter and exposed person substantially reduces the probability of successful transmission, even if masks only lower exposure viral load by ~ 50%. The model also predicts that moderately efficacious masks will lower exposure viral load tenfold among people who get infected despite masking, potentially limiting infection severity.
30 May
Schulz C, Wylezich C, Wernike K, Gründl M, Dangel A, Baechlein C, et al. Prolonged SARS-CoV-2 RNA shedding from therapy cat after cluster outbreak in retirement home. Emerg Inf Dis May 26, 2021 https://wwwnc.cdc.gov/eid/article/27/7/20-4670_article
Although an infected and asymptomatic therapy cat in a nursing home in Germany showed prolonged shedding of SARS-CoV-2 RNA up to day 21 after the first detection, genome sequencing found no further role of the cat in human infections on site.
29 May
Revollo B, Blanco I, Toro J, et al. Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial. Lancet Inf Dis May 27, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00268-1/fulltext
This RCT assessed the risk of COVID-19 transmission in an indoor mass-gathering live concert done under comprehensive safety measures, including same-day SARS-CoV-2 screening with Ag-RDTs, compulsory wearing of N95 face masks, and optimized air ventilation. The event, held in the Sala Apolo, Barcelona, Spain, on December 12, 2020, lasted for 5 h and included four performances: two DJ sessions and two live music acts. Participants were encouraged to sing and dance in the concert hall room, and no physical distancing was recommended. None of the 465 participants became infected, compared with two of the 495 people in the control arm.
24 May
Sowerby LJ, Nichols AC, Gibson R, et al. Assessing the Risk of SARS-CoV-2 Transmission via Surgical Electrocautery Plume. JAMA Surgery May 21, 2021. https://jamanetwork.com/journals/jamasurgery/fullarticle/2780434?resultClick=1
SARS-CoV-2 was not detectable in aerosol cautery plume (“surgical smoke”) generated from electrocautery under any of the conditions studied despite the high viral titers used.
22 May
Cheng Y, Ma N, Witt C, et al. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 20 May 2021. https://science.sciencemag.org/content/early/2021/05/19/science.abg6296
When people see images of millions of respiratory particles exhaled by talking or coughing, they may be afraid that simple masks cannot really protect them. This modeling study explains why wearing simple masks can indeed keep the number of inhaled viruses low. However, unfavorable conditions and the large variability of viral loads may lead to a virus-rich regime in certain indoor environments. In such environments, high efficiency masks and further protective measures like efficient ventilation should be used. The non-linear dependence of mask efficacy on airborne virus concentration also highlights the importance of combining masks with other preventive measures.
20 May
Prince SE, Chen H, Tong H, et al. Assessing the effect of beard hair lengths on face masks used as personal protective equipment during the COVID-19 pandemic. J Expo Sci Environ Epidemiol May 18, 2021. https://www.nature.com/articles/s41370-021-00337-1
Where is your shaving kit? In bearded men, KF94 and KN95 masks lost up to 40% of their fitted filtration efficiency (FFE). Although N95 respirators showed considerable variability among bearded men, they had the highest FFE for beard lengths of maximum 10 mm.
19 May
Decaro N, Vaccari G, Lorusso A, Lorusso E, De Sabato L, Patterson EI, et al. Possible human-to-dog transmission of SARS-CoV-2, Italy, 2020. Emerg Infect Dis May 12, 2021. https://doi.org/10.3201/eid2707.204959
Pet story, volume one: A female poodle, who was 1.5 years of age, lived with 4 family members in Bitonto, Italy. All five became infected. Full-length genome sequencing showed that the canine and human viruses were identical, suggesting human-to-animal transmission.
Colitti B, Bertolotti L, Mannelli A, et al. Cross-sectional serosurvey of companion animals housed with SARS-CoV-2–infected owners, Italy. Emerg Infect Dis May 11, 2021. 2021 Jul [date cited]. https://doi.org/10.3201/eid2707.203314
Pet story, volume two: Among animals living with SARS-CoV-2–infected owners, 20.4% (11/54) of cats and 3.2% (3/93) of dogs were seropositive. A 13-year-old female cat was hospitalized for a brachial cephalic thrombosis and a 3-year-old male cat was hospitalized for interstitial pneumonia.
4 May
Issakhov A, Zhandaulet Y, Omarova P et al. A numerical assessment of social distancing of preventing airborne transmission of COVID-19 during different breathing and coughing processes. Sci Rep May 3, 2021, 11, 9412. https://www.nature.com/articles/s41598-021-88645-2
Paper of the day. This study looked at the transport of droplets or particles generated by the respiratory system in a room during various scenarios. Results: Social distance of 2 m is sufficient for simple breathing for all cases (without ventilation and with ventilation). However, when coughing or sneezing, this distance is clearly not enough and it needs at least 5 m social distance in order not to get into the zone of exposure to these particles.
28 April
Amorim MR, Souza WM, Barros ACG Jr, Toledo-Teixeira DA, Bispo-dos-Santos K, Simeoni CL, et al. Respiratory viral shedding in healthcare workers reinfected with SARS-CoV-2, Brazil, 2020. Emerg Infect Dis. 2021 June [date cited]. https://doi.org/10.3201/eid2706.210558
Four cases of SARS-CoV-2 reinfection among HCWs. In each HCW, shedding of infectious viral particles was observed during both infection episodes.
Gonzalo EM, Martinez PP, Mahmud AS, et al. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 27 Apr 2021: eabg5298. DOI: https://science.sciencemag.org/content/early/2021/04/26/science.abg5298
Data from the capital of Chile, a highly segregated city: People living in municipalities with low socioeconomic status did not reduce their mobility during lockdowns as much as those in more affluent municipalities. Both test positivity rates and testing delays were much higher. There was a strong association between socioeconomic status and mortality, measured either by COVID-19 attributed deaths or excess deaths. Finally, infection fatality rates in young people were (1.7-3.1 fold) higher in low-income municipalities.
24 April
Mack CD, DiFiori J, Tai CG, et al. SARS-CoV-2 Transmission Risk Among National Basketball Association Players, Staff, and Vendors Exposed to Individuals With Positive Test Results After COVID-19 Recovery During the 2020 Regular and Postseason. JAMA Intern Med April 22, 2021. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2779287?resultClick=1
A negative PCR is not necessary for ending up quarantine. In this large retrospective cohort study of the 2020 NBA closed campus occupational health program, none of the 36/3648 (1%) participants who continued to test positive for SARS-CoV-2 (cycle threshold range 30-37) following discontinuation of isolation were infectious to others. Of note, this included 29 individuals who were engaged in at least 1480 person-days of indoor, unmasked contact events or situations during the period of persistent positive test results, supporting a time-based approach to discontinuation of isolation.
20 April
Allen JG, Ibrahim AM. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA April 16, 2021. https://jamanetwork.com/journals/jama/fullarticle/2779062
Some thoughts on far-field airborne transmission (defined as within-room but beyond 6 feet) of SARS-CoV-2. Air changes per hour and air filtration is a simple but important concept that could be deployed to help reduce risk from within-room but distant airborne transmission of SARS-CoV-2 and other respiratory infectious diseases. Healthy building controls like higher ventilation and enhanced filtration are a fundamental, but often overlooked, part of risk reduction strategies that could have benefit beyond the current pandemic.
19 April
Braun KM, Moreno GK, Buys A, et al. Viral sequencing reveals US healthcare personnel rarely become infected with SARS-CoV-2 through patient contact. Clinical Infectious Diseases April 15, 2021, ciab281. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab281/6226897?searchresult=1
Katarina Braun and colleagues from Madison, Wisconsin, US investigated SARS-CoV-2 infection clusters involving 95 HCP and 137 possible patient contact sequences. Strikingly, only 4/95 of HCP infections could be traced back to a patient source.
18 April
Sickbert-Bennett EE, Samet JM, Prince SE, et al. Fitted Filtration Efficiency of Double Masking During the COVID-19 Pandemic. JAMA Intern Med. 2021; https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2778913
Anthony Fauci is doing right when wearing two masks. This study compared the fitted filtration efficiency (FFE) of commonly available masks worn alone, doubled, or in combinations. Wearing a medical procedure mask underneath a cloth mask provided the best improvement to FFE of all the combinations evaluated.
17 April
Cheng VC, Fung SC, Siu GT, et al. Nosocomial outbreak of COVID-19 by possible airborne transmission leading to a superspreading event. Clinical Infectious Diseases 14 April 2021. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab313/6225253?searchresult=1
A superspreading event involving 12 patients and 9 healthcare workers (HCWs) occurred within 4 days in 3 of 6 cubicles at an old-fashioned general hospital ward with no air exhaust system within the cubicles. Some evidence (however, no proof) that transmission was airborne.
16 April
Dietrich WL, Bennett JS, Jones BW, Hosni MH. Laboratory Modeling of SARS-CoV-2 Exposure Reduction Through Physically Distanced Seating in Aircraft Cabins Using Bacteriophage Aerosol — November 2020. MMWR Morb Mortal Wkly Rep. ePub: 14 April 2021. DOI: https://www.cdc.gov/mmwr/volumes/70/wr/mm7016e1.htm?s_cid=mm7016e1_w
Boeing, listen up! Based on laboratory modelling of exposure (not transmission!) to SARS-CoV-2 on single-aisle and twin-aisle aircraft, exposures in scenarios in which the middle seat was vacant were reduced by 23% to 57%, compared with full aircraft occupancy, depending upon the model.
Spinelli MA, Rutherford G, Gandhi M. Lowering SARS-CoV-2 viral load might affect transmission but not disease severity in secondary cases – Authors’ reply. Lancet April 14, 2021. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00210-3/fulltext
Some comments about a potential relationship between non-pharmaceutical interventions (NPIs) and the viral inoculum as well as the impact of reduced inoculum on COVID-19 severity.
13 April
Park M, Pawliuk C, Nguyen T, et al. Determining the communicable period of SARS-CoV-2: A rapid review of the literature, March to September 2020. Euro Surveill. 2021;26(14):pii=2001506. https://doi.org/10.2807/1560-7917.ES.2021.26.14.2001506
After reviewing 160 studies, the authors conclude (not surprisingly) significant heterogeneity in reported data. The median duration after symptom onset that virus was successfully isolated was 9 days (IQR: 2.25; range: 2–21), while the corresponding median value for longest duration until viral clearance by RT-PCR was 26 days (IQR: 16.8; range: 8–63).
9 April
Katelaris AL, Wells J, Clark P, Norton S, Rockett R, Arnott A, et al. Epidemiologic evidence for airborne transmission of SARS-CoV-2 during church singing, Australia, 2020. Emerg Infect Dis. 2021 Jun. https://wwwnc.cdc.gov/eid/article/27/6/21-0465_article
An outbreak among church attendees after an infectious chorister sang at multiple services. 12 secondary case patients. Video recordings showed that case patients were seated in the same section, > 15m from the primary case patient, without close physical contact, suggesting airborne transmission. Thank god there were no deaths, although 3 case patients were hospitalized, including 2 who required intensive care.
Westhölter D, Taube C. SARS-CoV-2 outbreak in a long-term care facility after vaccination with BNT162b2. Clin Inf Dis April 7, 2021, ciab299. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab299/6213878?searchresult=1
What a disaster. In early January 2021, 73/76 (96%) residents and about 90% of the employees in an elderly care home in North-Rhine Westfalia, Germany, received a first dose of BNT162b2 (BioNTech/Pfizer). SARS-CoV-2 rapid antigen tests were all negative among residents and participating employees the day before. However, a member of the mobile vaccination team as well as an employee reported respiratory symptoms one and four days after vaccination respectively and tested positive for SARS-CoV-2 by PCR. Overall case fatality rate was 9/26 (35%).
8 April
Sami S, Turbyfill CR, Daniel-Wayman S, et al. Community Transmission of SARS-CoV-2 Associated with a Local Bar Opening Event — Illinois, February 2021. MMWR Morb Mortal Wkly Rep. ePub: 5 April 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7014e3.htm?s_cid=mm7014e3_w
February 2021 (no, not 2020): an opening event at a 2800-sq-ft bar in a rural Illinois county, accommodating approximately 100 persons. The event occurred indoors, with no air flow from outside. Cheers! The results: 46 cases, hospitalization of a long-term facility resident, and a school closure affecting 650 children. Two lessons learnt: first, an outbreak in a bar not only affects patrons and employees but can affect an entire community. Second, please forget “living with the virus” (before the event, average daily COVID-19 incidence had been 41 cases per 100,000 persons in the county).
6 April
Reukers DF, van Boven M, Meijer A, et al. High infection secondary attack rates of SARS-CoV-2 in Dutch households revealed by dense sampling. Clin Inf Dis April 2, 2021, ciab237, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab237/6209401?searchresult=1
In this Dutch study conducted in March/April 2020, estimated infection of SARs were higher than reported in earlier household studies, presumably owing to a dense sampling protocol: 35% (95%CI: 24%-46%) in children and 51% (95%CI: 39%-63%) in adults.
26 March
Forbes H, Morton CE, Bacon S, et al. Association between living with children and outcomes from covid-19: OpenSAFELY cohort study of 12 million adults in England. BMJ 2021; 372. Full text: https://doi.org/10.1136/bmj.n628
Does the risk of SARS-CoV-2 infection and outcomes of COVID-19 differ between adults living with and without children? In the second UK wave (1 September to 18 December 2020), the authors found an increased risk of SARS-CoV-2 infection and COVID-19-related hospital admission for adults living with children of all age groups in the second wave. For adults aged over 65 years, they also found an increased risk of infection associated with living with children of any age and of ICU admission and death from COVID-19 for those living with children aged 0-18 years.
Huang N, Pérez P, Kato T, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med (2021). https://doi.org/10.1038/s41591-021-01296-8
The oral cavity is an important site for SARS-CoV-2 infection. The authors discuss saliva as a potential transmission route.
25 March
Plesner Lyngse F, Mølbak K, Træholt Frank K, et al. Association between SARS-CoV-2 Transmission Risk, Viral Load, and Age: A Nationwide Study in Danish Households. medRxiv 2021, posted 5 March. Full-text: https://doi.org/10.1101/2021.02.28.21252608
Should age have a higher impact than Ct value on the risk of SARS-CoV-2 transmission? This is the conclusion of a study by Frederik Lyngse et al. who found an almost linearly increasing transmission risk with age of the primary cases for adults (≥ 20 years) and negatively for children (< 20 years). They also found that the risk of SARS-CoV-2 transmission was negatively associated—approximately linear—with the Ct values of the tested primary cases. However, even for relatively high Ct values, the risk of transmission was not negligible, e.g. for primary cases with a Ct value of 38, the authors found a transmission risk of 8%.
24 March
Paper of the Day
Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol 2021, published 22 March. Full text: https://doi.org/10.1038/s41579-021-00535-6
23 March
Couthinho M, Darcie Marquitti FM, Souto Ferreira L, et al. Model-based estimation of transmissibility and reinfection of SARS-CoV-2 P.1 variant. medRxiv 2021, posted 9 March. Full-text: https://doi.org/10.1101/2021.03.03.21252706
From December, 2020 to February, 2021, Manaus was devastated by four times more COVID-19 cases compared to its previous peak (April, 2020). The probable culprit: the new P.1 variant. In this pre-print, the authors describe the transmissibility of P.1. to be about 2.5 times higher compared to the historical variant in Manaus. The probability of re-infection by P.1 seems to be low, though: 6.4% (95% CI: 5.7–7.1%).
Ferasin L, Fritz M, Ferasin H, et al. Myocarditis in naturally infected pets with the British variant of COVID-19. bioRxiv 2021, posted 18 March. Full-text: https://doi.org/10.1101/2021.03.18.435945
Given the enhanced infectivity and transmissibility of the B.1.1.7 variant, is there a risk that companion animals may play a more significant role in SARS-CoV-2 outbreak dynamics than previously appreciated? The authors report the first cases of infection of domestic cats and dogs by the British B.1.1.7 variant. They also discovered that many owners and handlers of these pets had developed COVID-19 respiratory symptoms 3-6 weeks before their pets became ill.
15 March
Jordan I, Fernandez de Sevilla M, Bassat Q, et al. Transmission of SARS-CoV-2 infection among children in summer schools applying stringent control measures in Barcelona, Spain. Clinical Infectious Diseases, 12 March 2021, ciab227, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab227/6168543
Among the more than 2000 repeatedly screened participants, the transmission rate of SARS-CoV-2 infection among children attending school-like facilities under strict prevention measures was lower than that reported for the general population.
Soriano-Arandes A, Gatell A, Serrano P, et al. Household SARS-CoV-2 transmission and children: a network prospective study. Clinical Infectious Diseases 12 March 2021, ciab228, https://doi.org/10.1093/cid/ciab228
Same direction: children are unlikely to cause household COVID-19 clusters or be major drivers of the pandemic even if attending school. Among 1040 COVID-19 patients < 16 years, more than 70% (756) were secondary to an adult infection, while only 7.7% (80) were index cases.
14 March
Hensley MK, Bain WG, Jacobs J, et al. Intractable Coronavirus Disease 2019 (COVID-19) and Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Replication in a Chimeric Antigen Receptor-Modified T-Cell Therapy Recipient: A Case Study. Clin Infect Dis 2021. Full-text: https://doi.org/10.1093/cid/ciab072
Prolonged transmission from immunosuppressed patients is possible. A chimeric antigen receptor-modified T cell therapy recipient developed severe coronavirus disease 2019, intractable RNAemia, and viral replication lasting > 2 months. Deep sequencing revealed multiple sequence variants consistent with intra-host viral evolution.
13 March
Klompas M, Baker MA, Griesbach D, et al. Transmission of SARS-CoV-2 from asymptomatic and presymptomatic individuals in healthcare settings despite medical masks and eye protection. Clinical Infectious Diseases, 11 March 2021. ciab218, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab218/6168040?searchresult=1
Three instances of SARS-CoV-2 transmission in Boston, despite medical masks and eye protection, including one transmission despite both parties being masked. Whole genome sequencing confirmed perfect homology between source and exposed persons’ viruses in all cases. These findings teach the importance of not relying upon medical masks and eye protection alone.
Pan X, Li X, Kong P, et al. Assessment of Use and Fit of Face Masks Among Individuals in Public During the COVID-19 Pandemic in China. JAMA Netw Open March 11, 2021, 4(3):e212574. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2777405?resultClick=1
“Wear a mask.” If it were only that simple, even in China! For this study, during July and August 2020, 6003 (!) people wearing masks were assessed at public places, such as markets, train stations, airports, hospitals, and schools in Beijing, Yunnan, Shanxi, and Jiangsu. Face mask airtightness was commonly suboptimal, mostly secondary to gaps at the upper face mask edge. Sealing the upper face mask edge with an adhesive tape was associated with significantly improving the airtightness of commonly used face masks.
10 March
Paul LA, Daneman N, Brown KA, et al. Characteristics associated with household transmission of SARS-CoV-2 in Ontario, Canada: A cohort study. Clinical Infectious Diseases 05 March 2021, ciab186, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab186/6159706
Among 26,714 cases of COVID-19 residing in 21,226 households, longer testing delays (≥ 5 days versus 0 days, OR = 3.02) and male gender (OR = 1.28) were associated with greater odds of household secondary transmission, as well as (not surprisingly) larger average family size and a higher proportion of households with multiple persons per room.
7 March
Dinklage F, Ehmann A, Erdmann E, et al. Why Is the Risk of Coronavirus Transmission so High Indoors? Die Zeit 2020, published 26 November. Full-text: https://www.zeit.de/wissen/gesundheit/2020-11/coronavirus-aerosols-infection-risk-hotspot-interiors
Whenever people gather in closed spaces, the infection risk climbs. This interactive tool shows how the coronavirus spreads. Find out how safe your environment is. See also the German version.
5 March
Shumsky RA, Debo L, Lebeaux RM, et al. Retail store customer flow and COVID-19 transmission. PNAS February 26, 2021. https://doi.org/10.1073/pnas.2019225118
Nice modelling study on how customer flows in retail stores affect the rate of COVID-19 transmission. The model calculates the disease transmission rate in a retail environment and determines how the rate depends on the customer flow policy (one-way versus two-way), the travel speed distribution, and store size. Restricting customer flow to one-way movement is highly effective if direct exposure is the dominant mode of transmission. However, it is not effective if more indirect (“wake”) exposure dominates.
Schumm MA, Hadaya JE, Mody N, et al. Filtering Facepiece Respirator (N95 Respirator) Reprocessing: A Systematic Review. JAMA. March 3, 2021; doi: 10.1001/jama.2021.2531. https://jamanetwork.com/journals/jama/fullarticle/2777342?resultClick=1
Max A. Schumm and colleagues have reviewed 42 studies (through January 31, 2021) that examined 65 types of masks. Ultraviolet germicidal irradiation, vaporized hydrogen peroxide, moist heat, and microwave-generated steam processing effectively sterilized N95 respirators and retained filtration performance. Ultraviolet irradiation and vaporized hydrogen peroxide damaged respirators the least.
4 March
Lubrano R, Bliose S, Testa A, et al. Assessment of Respiratory Function in Infants and Young Children Wearing Face Masks During the COVID-19 Pandemic. JAMA Netw Open March 2, 2021. 4(3):e210414. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2776928
Are surgical masks associated with episodes of oxygen desaturation or respiratory distress among children? This study from Italy says no. Among 47 children (22 aged 24 months or younger!) the use of facial masks was not associated with significant changes in Sao2 or Petco2.
Wilmes P, Zimmer J, Schulz J, et al. SARS-CoV-2 transmission risk from asymptomatic carriers: Results from a mass screening programme in Luxembourg. Lancet Regional Health February 27, 2021. DOI: https://doi.org/10.1016/j.lanepe.2021.100056
To accompany the lifting of COVID-19 lockdown measures, Luxembourg implemented a mass screening (MS) program. Based on a participation of 49% amongst the resident population and 22% amongst cross-border workers, the MS program allowed identification of 1099 cases corresponding to 26% of positive cases related to an early summer epidemic wave. Among the index cases, 567 (67%) reported symptoms at the time of being informed of their positive test result (these may have been pre-symptomatic at the time of the test), whereas 283 (33%) were asymptomatic. Asymptomatic individuals had significant secondary attack rates, both in households and among close contacts, but these were lower compared to those for symptomatic cases.
1 March
Metlay JP, Haas JS, Soltoff AE, Armstrong KA. Household Transmission of SARS-CoV-2. JAMA Netw Open. 2021 Feb 1;4(2):e210304. PubMed: https://pubmed.gov/33635324. Full-text: https://doi.org/10.1001/jamanetworkopen.2021.0304
In this large hospital and ambulatory care network based in Boston, US, 7262 index cases were linked to 17,917 additional at-risk individuals assigned to the same addresses. Overall household infection risk was 10.1%. Independent factors significantly associated with higher transmission risk included age greater than 18 years and multiple comorbid conditions (adjusted OR for individuals with hypertension, 1.93). In sensitivity analyses limiting the maximum size of the household to as small as 2 persons, the calculated transmission risk increased to only 13.8%.
Kraay ANM, Hayashi MAL, Berendes DM, Sobolik JS, Leon JS, Lopman BA. Risk for fomite-mediated transmission of SARS-CoV-2 in child daycares, schools, nursing homes, and offices. Emerg Infect Dis. February 24, 2021. https://wwwnc.cdc.gov/eid/article/27/4/20-3631_article
SARS-CoV-2 can persist on surfaces, suggesting possible surface-mediated transmission of this pathogen. Using a transmission model to explore the potential for fomite transmission without other pathways, Alicia N.M. Kraay from Atlanta and colleagues found that fomites might be a substantial source of transmission risk, particularly in schools and child daycares. Combining surface cleaning and decontamination with mask wearing can help mitigate this risk.
28 February
Davis AC, Zee M, Clark AD, et al. Computational Fluid Dynamics Modeling of Cough Transport in an Aircraft Cabin. medRxiv 2021, posted 17 February. Full-text: https://doi.org/10.1101/2021.02.15.431324
Track particles released by coughing from a passenger seated in different seats on a Boeing 737 aircraft. The authors report that 80% of the particles were removed from the cabin in 1.3 to 2.6 minutes, depending on conditions, and 95% of the particles were removed in 2.3 to 4.5 minutes. We know from other reports that this is not sufficient to prevent SARS-CoV-2 transmission onboard aircraft. Remember the paper we presented on 23 February: Wang Z, Galea ER, Grandison A, et al. Inflight Transmission of COVID-19 Based on Experimental Aerosol Dispersion Data. Journal of Travel Medicine, February 19, 2021. taab023, https://doi.org/10.1093/jtm/taab023
27 February
Marcus JE, Frankel DN, Pawlak MT, et al. Risk Factors Associated With COVID-19 Transmission Among US Air Force Trainees in a Congregant Setting. JAMA Netw Open. 2021 Feb 1;4(2):e210202. PubMed: https://pubmed.gov/33630090. Full-text: https://doi.org/10.1001/jamanetworkopen.2021.0202
Basic military training is the first step in the transition of a civilian to an enlisted member of the US Air Force. It brings together more than 39,000 trainees every year from around the US and represents an “ideal” setting to assess symptoms and lab values of a young, healthy population living in congregant-setting cohorts in a controlled environment. Among 10,613 US Air Force basic trainees in 263 cohorts, 403 trainees (3%) received a COVID-19 diagnosis in 129 cohorts (49%). Of these, 204 (51%) were symptomatic, and 199 (49%) were asymptomatic. Median cycle threshold values were lower in symptomatic trainees compared with asymptomatic trainees (21.2 vs 34.8). Cohorts with infection clusters were predominantly men, had more symptomatic trainees, and had more symptoms per patient compared with cohorts without clusters.
25 February
Spinelli MA, Gliden DV, Gennatas ED. Importance of non-pharmaceutical interventions in lowering the viral inoculum to reduce susceptibility to infection by SARS-CoV-2 and potentially disease severity. Lancet Inf Dis February 22, 2021. Full-text: https://doi.org/10.1016/S1473-3099(20)30982-8
Matthew Spinelli and colleagues argue that even as safe and effective vaccines are being rolled out, non-pharmaceutical interventions (including social distancing, mask wearing, and improved ventilation) will continue to play an essential role in suppressing SARS-CoV-2 transmission until equitable and widespread vaccine administration has been completed. In this personal viewpoint, they review the influence of the viral inoculum on disease susceptibility for several human pathogens and the preliminary data available for SARS-CoV-2.
23 February
Paper of the Day
Wang Z, Galea ER, Grandison A, et al. Inflight Transmission of COVID-19 Based on Experimental Aerosol Dispersion Data. Journal of Travel Medicine, February 19, 2021. taab023, https://doi.org/10.1093/jtm/taab023
No nuts on planes. This elegant analysis demonstrated that while there is a significant reduction in aerosol concentration due to the nature of the cabin ventilation and filtration system, this does not necessarily mean that there is a low probability or risk of in-flight infection. Main results: 1. The economy cabin exhibits the highest probability of infection. 2. Average risk (without masks) for a 2-hour flight in a B777–200 aircraft ranges from 0.1% to 2.5% and for a 12-hour flight from 0.8% to 10.8%, respectively. 3. If all passengers wear face masks throughout the 12-hour flight, the average infection probability can be reduced by approximately 73%/32% for high/low efficiency masks. 4. If face masks are worn by all passengers except during a one-hour meal service, the average infection probability is increased by 59%/8% compared to the situation where the mask is not removed. Bottom line: Don’t remove your KN95 mask. No nuts, no meals. And better forget your frequent flyer status as long as you are unvaccinated (actually, forget it anyway, we’ll get warm here up north and Zoom works fine).
Bender JK, Brandl M, Höhle M, Buchholz U, Zeitlmann N. Analysis of asymptomatic and presymptomatic transmission in SARS-CoV-2 outbreak, Germany, 2020. Emerg Infect Dis February 18, 2021 Apr [date cited]. https://wwwnc.cdc.gov/eid/article/27/4/20-4576_article
Jennifer K. Bender and colleagues determined secondary attack rates (SAR) among close contacts of 46 symptomatic and 7 asymptomatic patients from Southern Germany. Little to no transmission occurred from asymptomatic case-patients. Pre-symptomatic transmission was more frequent than symptomatic transmission.
22 February
Kristiansen MF, Heimustovu BH, á Borg S, Mohr TH, Gislason H, Møller LF, et al. Epidemiology and clinical course of first wave coronavirus disease cases, Faroe Islands. Emerg Infect Dis. 2021 Mar [date cited]. https://doi.org/10.3201/eid2703.202589
The Faroe Islands are a unique place to investigate the effects of COVID-19. Because of large scale testing in the country, few unrecorded cases are expected. Mapping the transmission chains of COVID-19 on the islands reveals that most cases infected few or no secondary contacts, whereas 3 superspreading cases set off long, aggressive chains that led to most of the identified secondary locally transmitted cases.
19 February
Kissler S, Fauver JR, Mack C, et al. Densely sampled viral trajectories suggest longer duration of acute infection with B.1.1.7 variant relative to non-B.1.1.7 SARS-CoV-2. dash.harvard.edu 2021, accessed 17 February. Full-text: https://dash.harvard.edu/handle/1/37366884
B.1.1.7 may cause longer infections with similar peak viral concentration compared to non-B.1.1.7 variants. This extended duration may contribute to B.1.1.7 SARS CoV-2’s increased transmissibility. Yonatan Grad, Stephen Kissler and colleagues assessed longitudinal PCR tests performed in a cohort of 65 individuals infected with SARS-CoV-2 undergoing daily surveillance testing, including seven infected with B.1.1.7.
| B.1.1.7 | non-B.1.1.7 | |
| Mean duration of the proliferation phase |
5,3 days(2,7, 7,8)
(90% credible interval) |
2,0 days(0,7, 3,3) |
| Mean duration of the clearance phase |
8,0 days(6,1, 9,9) | 6,2 days(5,1, 7,1) |
| Mean overall duration of infection (proliferation + clearance phase) |
13,3 days(10,1, 16,5) | 8,2 days(6,5, 9,7) |
| Peak viral concentration | 19,0 Ct(15,8, 22,0) | 20,2 Ct(19,0, 21,4) |
| log10 RNA copies/ml | 8,5(7,6, 9,4) | 8,2(7,8, 8,5) |
Tan ZP, Silwal L, Bhatt SP, et al. Experimental characterization of speech aerosol dispersion dynamics. Sci Rep 11, 3953 (2021). Full-text: https://www.nature.com/articles/s41598-021-83298-7
Relative to sneezing and coughing, non-symptomatic aerosol-producing activities such as speaking are highly understudied. Here, Vrishank Raghav, Zu Puayen Tan and colleagues of Auburn University, US, delve into the details of jet phases and puff phases. One of their conclusions: speaking may represent a higher transmission risk than coughs and sneezes. Sneezing and coughing are singular events with a plume-front that passes by quickly, whereas speaking is a prolonged activity continuously producing plumes of aerosols.
Shriner SA, Ellis JW, Root JJ, Roug A, Stopak SR, Wiscomb GW, et al. SARS-CoV-2 exposure in escaped mink, Utah, USA. Emerg Infect Dis. 2021 Mar. Full-text: https://doi.org/10.3201/eid2703.204444
Free-range mink, presumed domestic escapees, exhibited high antibody titers, suggesting a potential SARS-CoV-2 transmission pathway to native wildlife. Interactions or shared resources between escaped mink and wild mink or other wildlife species represent potential transmission pathways for spillover of SARS-CoV-2 into wildlife and could lead to health consequences or establishment of new reservoirs in susceptible wildlife
Illingworth C, Hamilton W, Warne B, et al. Superspreaders drive the largest outbreaks of hospital onset COVID-19 infection. OSF Preprints 2021, posted 15 February. Full-text: https://osf.io/wmkn3/
Chris Illingworth et al. provides a detailed retrospective analysis of nosocomial SARS-CoV-2 transmission. Their data were consistent with a pattern of superspreading, where 20% of individuals caused 80% of transmission events.
17 February
Marr L, Miller S, Prather K, et al. FAQs on Protecting Yourself from COVID-19 Aerosol Transmission. Cloud 2021, Version 1.87, 9 December. Full-text: https://tinyurl.com/FAQ-aerosols
Excellent summary of aerosol protection.
Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: Using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. 2021 Feb 11;17(2):e1008559. PubMed: https://pubmed.gov/33571188. Full-text: https://doi.org/10.1371/journal.pcbi.1008559
The fact that the fraction of children among confirmed cases has been found to be low in many countries can be accounted for by two (non-exclusive) hypotheses: (1) Children display milder symptoms than adults when infected, so are less likely to be tested in a typical testing policy triggered by symptoms, (2) Children are less susceptible to infection than adults. The answer by Dattner and all? Both!
16 February
Avadhanula V, Nicholson EG, Ferlic-Stark L, et al. Viral load of SARS-CoV-2 in adults during the first and second wave of COVID-19 pandemic in Houston, TX: the potential of the super-spreader. J Infect Dis 2021, published 15 February. Full-text: https://doi.org/10.1093/infdis/jiab097
Pedro Piedra, Vasanthi Avadhanula and colleagues describe the first two waves of the SARS-CoV-2 pandemic in Houston, US. They observed an increase in the weekly median viral load that predated the onset of each wave by approximately two weeks. A small group of individuals with extremely high and high viral load represented 7,1% and 20,8%, respectively, of the RT-PCR positives in this study. The authors believe that these individuals’ characteristics could be consistent with the super-spreader phenomenon. Greater awareness of the social dynamics of these individuals would be needed to understand the spread of SARS-CoV-2.
13 February
Edwards DA, Ausiello D, Salzman J, et al. Exhaled aerosol increases with COVID-19 infection, age, and obesity. PNAS 2021, published 23 February. Full-text: https://www.pnas.org/content/118/8/e2021830118
David Edwards et al. report studies of exhaled aerosol suggesting that a critical factor in SARS-CoV-2 superspreading events is the propensity of certain individuals to exhale large numbers of small respiratory droplets. Their findings indicate that the capacity of airway lining mucus to resist breakup on breathing varies significantly between individuals, with a trend to increasing with the advance of COVID-19 infection and body mass index multiplied by age (i.e., BMI-years).
11 February
Lachassinne E, de Pontual L, Caseris M, et al. SARS-CoV-2 transmission among children and staff in daycare centres during a nationwide lockdown in France: a cross-sectional, multicentre, seroprevalence study. Lancet Child Adolesc Health, published 8 February. Full-text: https://www.thelancet.com/journals/lanchi/article/PIIS2352-4642(21)00024-9/fulltext
During a nationwide lockdown in France in June 2020, the authors enrolled 327 children (mean age 1,9 [SD 0,9] years; range 5 months to 4,4 years), 197 daycare center staff (mean age 40 [12] years), and 164 adults in a comparator group. An exploratory analysis suggested that seropositive children were more likely than seronegative children to have been exposed to an adult household member with laboratory-confirmed COVID-19. Intra-family transmission seemed more plausible than transmission within daycare centers.
Liu H, He S, Shen L, Hong J. Simulation-based study of COVID-19 outbreak associated with air-conditioning in a restaurant featured. Physics of Fluids 33, 023301 (2021). Full-text: https://doi.org/10.1063/5.0040188
COVID-19 is transmitted via droplets and virus-carrying aerosols. Here, Han Liu et al. conducted a systematic computational fluid dynamics (CFD)-based investigation of indoor airflow and the associated aerosol transport in a restaurant setting. They provide a spatial map of the airborne infection risk in the restaurant under different air conditioning and thermal settings.
6 February
Madera S. Crawford E, Langelier C, et al. Nasopharyngeal SARS-CoV-2 viral loads in young children do not differ significantly from those in older children and adults. Sci Rep 11, 3044 (2021). Full-text: https://doi.org/10.1038/s41598-021-81934-w
This study of 5544 children and adults did not demonstrate higher nasopharyngeal viral loads in children under five years of age.
5 February
Paper of the Day
Letizia AG, Ge Y, Vangeti S, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. medRxiv 2021, posted 29 January. Full-text: https://doi.org/10.1101/2021.01.26.21250535
High transmission rate in a US Marine camp despite a 2-week home quarantine plus another 2-week quarantine at the camp. Would you have placed a bet on a 48% transmission rate after another 6 weeks (1079 out of 2247 young recruits)? The authors caution that “the crowded living conditions, demanding regimen and requirement for personal contact during basic training despite the pandemic leads not only to an increased risk for respiratory epidemics, but also potentially to higher exposure levels. The close quarters and constant contact among recruits that are needed for team building allows a viral infection to rapidly proliferate within a unit. The physically and mentally demanding training environment may also suppress immunity. These conditions may contribute to the high infection rate we observed during the six-week study period. These factors are not typically present in the civilian community.”
The worst is yet to come, though. A total of 19 out of 189 (10.1%, 1.1 cases per person-year) initially seropositive participants had at least one positive SARS-CoV-2 PCR result during the six-week study period. In other words: in crowded living conditions, the re-infection rate with SARS-CoV-2 can be quite high. Bad news for ‘herd immunologists’.
4 February
Paper of the Day
Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 2021, published 2 February. Full-text: https://doi.org/10.1016/S1473-3099(20)30985-3
A ground-breaking study! Oriol Mitjà, Michael Marks and colleagues found that increasing viral load values in nasopharyngeal swabs of patients with COVID-19 were associated with a greater risk of transmission, measured by SARS-CoV-2 PCR positivity among contacts, and with a higher risk of transmission in a household environment compared with other indoor situations. Read also the comment by Cornelissen L. Understanding the drivers of transmission of SARS-CoV-2. Lancet Infect Dis 2021, published 2 February. Full-text: https://doi.org/10.1016/S1473-3099(21)00005-0
3 February
Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. Ebiomedicine 2021, published 1 February. Full-text: https://doi.org/10.1016/j.ebiom.2021.103230
No SARS-CoV-2 transmission from individuals that remain SARS-CoV-2 PCR positive by pharnygeal swab weeks after recovery. The authors enrolled 203 post-symptomatic participants with a previous RT-PCR-verified SARS-CoV-2 infection. At time point 1, a median of 23 days (range 15–44) after recovery, 26 individuals (12,8%) were PCR positive. At time point 2, 90 days (median, range 85–105) after recovery, 5 (5,3%) were positive. The persistent PCR positive group however, had SARS-CoV-2-specific CD8 T cell responses of significantly increased breadth and magnitude. Contact tracing among persistent PCR positive individuals revealed zero new COVID-19 diagnoses among 757 close contacts. Persistent PCR positive individuals are not contagious at the post-symptomatic stage of the infection.
31 January
Transmission
Atherstone C, Siegel M, Schmitt-Matzen E, et al. SARS-CoV-2 Transmission Associated with High School Wrestling Tournaments — Florida, December 2020–January 2021. MMWR Morb Mortal Wkly Rep 2021;70:141–143. DOI: http://dx.doi.org/10.15585/mmwr.mm7004e4
December 2020? Too soon for news about SARS-CoV-2 to have spread through Florida, USA. A total of 130 wrestlers, coaches, and referees attended the tournaments. The results: 54 (41,5%) of the 130 tournament attendees received testing, and 38 cases of SARS-CoV-2 infection were identified. The minimum attack rate was 30,2%.
30 January
da Silva Francisco R, Benittes F, Lamarca AP, et al. Pervasive transmission of E484K and emergence of VUI-NP13L with evidence of SARS-CoV-2 co-infection events by two different lineages in Rio Grande do Sul, Brazil. medRxiv 2021, posted 26 January. Full-text: https://doi.org/10.1101/2021.01.21.21249764
Fernando Rosado Spilki, Ronaldo da Silva Francisco and colleagues report two co-infection events caused by the simultaneous occurrence of B.1.1.28 (E484K) and other lineages. Both patients had typical mild to moderate flu-like symptoms with favorable outcomes after disease, no required hospitalization and spontaneous recovery. The possibility of co-infection by E484K adds a new factor to the complex interaction between immune response systems and SARS-CoV-2 Spike mutations.
26 January
Tönshoff B, Müller B, Elling R, et al. Prevalence of SARS-CoV-2 Infection in Children and Their Parents in Southwest Germany. JAMA Pediatr January 22, 2021. Full-text: https://doi.org/10.1001/jamapediatrics.2021.0001
In a population-based sample in southwest Germany, this large-scale, multi-center, cross-sectional investigation of 4,964 participants accurately determined anti–SARS-CoV-2 seropositivity. The estimated SARS-CoV-2 seroprevalence was low in parents (1.8%) and 3-fold lower in children aged 1 to 10 years (0.6%), indicating that young children do not play a key role in SARS-CoV-2 spreading during the current pandemic (or their parents don’t hug them enough! J).
20 January
Paper of the Day
Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis 2021, published 18 January. Full-text: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30981-6/fulltext
Within households, children and adolescents are less susceptible to SARS-CoV-2 infection but may be more infectious than older individuals. This is the message by Shun-Qing Xu, Fang Li and colleagues who analyzed 27.101 households with 29.578 primary cases and 57.581 household contacts. The secondary attack rate was estimated at 15·6% (95% CI 15·2–16·0), assuming a mean incubation period of 5 days and a maximum infectious period of 22 days. Pre-symptomatic cases were more infectious and individuals with asymptomatic infection less infectious than symptomatic cases.
19 January
Mathai V, Das A, Bailey JA, Breuer K. Airflows inside passenger cars and implications for airborne disease transmission. Sci Adv. 2020 Dec 4;7(1):eabe0166. PubMed: https://pubmed.gov/33277325. Full-text: https://doi.org/10.1126/sciadv.abe0166
Are you afraid of driving with friends and family in your car because you remember that the transmission of SARS-CoV-2 is facilitated by exhaled droplets and aerosols suspended in air for extended periods of time? Then study the complex fluid dynamics during everyday commutes. The authors also reveal non-intuitive ways in which open windows can either increase or suppress airborne transmission. The preliminary recommendation by Varghese Mathai and colleagues: open the two windows farthest from the occupants (front right and rear left, respectively) which would give better protection to the passenger. See also the press article by Anthes E. How to (Literally) Drive the Coronavirus Away. The New York Times 2021, published 16 January. Full-text: https://www.nytimes.com/2021/01/16/health/coronavirus-transmission-cars.html
Samet JM, Prather K, Benjamin G, et al. Airborne Transmission of SARS-CoV-2: What We Know. Clin Infect Dis. 2021 Jan 18:ciab039. PubMed: https://pubmed.gov/33458756. Full-text: https://doi.org/10.1093/cid/ciab039
This perspective examines the potential for airborne transmission of SARS-CoV-2 using a source-to-dose framework that begins with generation of virus-containing droplets and aerosols and ends with the virus depositing in the respiratory tract of a susceptible individual. The authors ask four critical questions and summarize what we know (accepted manuscript).
Shah K, Kandre Y, Mavalankar D. Secondary attack rate in household contacts of COVID-19 Paediatric index cases: a study from Western India. J Public Health (Oxf). 2021 Jan 18:fdaa269. PubMed: https://pubmed.gov/33454742. Full-text: https://doi.org/10.1093/pubmed/fdaa269
The secondary attack rate (SAR) in household contacts of pediatric index cases may be as low as 1,7%. In this study from Gujarat, India, SAR was closely associated with the family size of the index cases. The authors conclude that home quarantine should be advocated for in smaller families with appropriate isolation facilities. Next week: what to do in larger families. (JOKE!)
16 January
van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021 Jan 11;12(1):267. PubMed: https://pubmed.gov/33431879. Full-text: https://doi.org/10.1038/s41467-020-20568-4
Quantitative RNA viral load assays and serological assays might be used in testing based strategies to discontinue or de-escalate infection prevention and control precautions. This is the result of a study by Jeroen van Kampen and colleagues who report that infectious viral shedding is detected by virus cultures in 23 of 129 patients (17,8%) hospitalized with COVID-19. Of these, 89 patients (69,0%) were admitted to intensive care and the remaining 40 (31,0%) admitted to medium care. The median duration of shedding infectious virus was 8 days post-onset of symptoms (IQR 5–11) and dropped below 5% after 15,2 days post-onset of symptoms (95% confidence interval (CI) 13,4–17,2). Viral loads above 7 log10 RNA copies/mL were independently associated with isolation of infectious SARS-CoV-2 from the respiratory tract.
Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open. 2021 Jan 4;4(1):e2035057. PubMed: https://pubmed.gov/33410879. Full-text: https://doi.org/10.1001/jamanetworkopen.2020.35057
The identification and isolation of persons with symptomatic COVID-19 alone might not control the ongoing spread of SARS-CoV-2. This is the conclusion of a decision model by Jay Butler, Michael Johansson and colleagues who assessed multiple scenarios for the infectious period and the proportion of transmission from individuals who never had COVID-19 symptoms. Measures such as mask wearing and physical distancing might be needed to protect them and their communities for quite some time.
14 January
Kasloff SB, Leung A, Strong JE, et al. Stability of SARS-CoV-2 on critical personal protective equipment. Sci Rep 11, 984 (2021). Full-text: https://doi.org/10.1038/s41598-020-80098-3
Cotton provides the lowest environmental stability to SARS-CoV-2. When applied to 100% cotton fabric, the virus underwent rapid degradation and became undetectable by TCID50 assay within 24 h. However, viable SARS-CoV-2 was recovered after 21 days on plastic, 14 days on stainless steel, 7 days on nitrile gloves and 4 days on chemical resistant gloves.
Hwang SE, Chang JH, Bumjo O, Heo J. Possible Aerosol Transmission of COVID-19 Associated with an Outbreak in an Apartment in Seoul, South Korea, 2020. Int J Infect Dis. 2020 Dec 17:S1201-9712(20)32558-3. PubMed: https://pubmed.gov/33346125. Full-text: https://doi.org/10.1016/j.ijid.2020.12.035
Indoor aerosol aerosol transmission might be underappreciated. This is the suggestion by Jongho Heo, Seo Eun Hwang and colleague who investigated a cluster of 9 COVID-19 infections in an apartment building in Seoul, South Korea. The investigation found no other possible contact between the cases than the airborne infection through a single air duct in the bathroom. All infected cases (living from the 2nd to the 11th floor of the building) were found along two vertical lines of the building, and each line was connected through a single air duct in the bathroom for natural ventilation. The authors assume that the first infected person probably released the virus during a shower in the bathroom by coughing, breathing, singing, or flushing and that SARS-CoV-2 may have combined with water vapor and became aerosols in the humid environment.
13 January
Volz E, Mishra W, Chand M, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv 2021, posted 4 January. Full-text: https://doi.org/10.1101/2020.12.30.20249034
The new SARS-CoV-2 lineage B117 (B.1.1.7, also named VOC 202012/01), originated in England in late Summer to early Autumn 2020. Available data indicate a larger share of under 20-year-olds among reported B117 than non-B117 cases. B117 seems to have a substantial transmission advantage, with the estimated difference in reproduction numbers between B117 and non-B117 ranging between 0.4 and 0.7. Neil Ferguson, Erik Volz and colleagues note that these estimates of transmission advantage apply to a period where high levels of social distancing were in place in England; extrapolation to other transmission contexts therefore requires caution.
11 January
Swadi T, Geoghegan JL, Devine T, et al. Genomic Evidence of In-Flight Transmission of SARS-CoV-2 Despite Predeparture Testing. Emerg Infect Dis. 2021 Jan 5;27(3). PubMed: https://pubmed.gov/33400642. Full-text: https://doi.org/10.3201/eid2703.204714
SARS-CoV-2 transmission may occur on aircraft and the risk may be increased during long-distance flights. Joep de Ligt, Tara Swadi and colleagues from New Zealand describe an air travel cluster where among the 7 eventually-infected passengers, 2 were probably index case-patients infected before the flight, 4 were probably infected during the flight, and the remaining passenger was probably infected while in New Zealand under “managed isolation and quarantine”. All 7 passengers were seated in aisle seats within 2 rows of where the presumed index case-patient(s) were seated.
9 January
Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021 Jan 8;371(6525):172-177. PubMed: https://pubmed.gov/33172935. Full-text: https://doi.org/10.1126/science.abe5901
We know that humans can infect animals with SARS-CoV-2, for example, cats and dogs, hamsters and rabbits. Here, Oude Munnink and colleagues show that that mink-to-human transmission also occurred. See also the perspective by Zhou P, Shi ZL. SARS-CoV-2 spillover events. Science. 2021 Jan 8;371(6525):120-122. PubMed: https://pubmed.gov/33414206. Full-text: https://doi.org/10.1126/science.abf6097
7 January
Davies NG, Barnard RC, Jarvis CI, et al. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. medRxiv 2020, posted 26 December. Full-text: https://doi.org/10.1101/2020.12.24.20248822
Nicolas Davies et al. estimate that the novel SARS-CoV-2 variant emerged in southeast England in November 2020, is 56% more transmissible (95% credible interval across three regions 50-74%) than preexisting variants of SARS-CoV-2. The author did not find evidence that VOC 202012/01 resulted in greater or lesser severity of disease than pre-existing variants. However, the increase in transmissibility is likely to lead to a large increase in incidence, with COVID-19 hospitalizations and deaths projected to reach higher levels in 2021 than were observed in 2020.
6 January
ECDC 20201223. COVID-19 in children and the role of school settings in transmission – first update. ECDC 2020, published 23 December. Full-text: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission
An update on the role of children in the transmission of SARS-CoV-2 and the role of schools in the COVID-19 pandemic, based on experience in the EU from August through December 2020. The document also addresses transmission to and from staff in school settings, school-related mitigation measures including risk communication, testing, contact tracing and the efficacy of partial and full school closures.
Children’s Task and Finish Group. Update to 4th Nov 2020 paper on children, schools and transmission. UK Government 2020, published 17 December. Full-text: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/948617/s0998-tfc-update-to-4-november-2020-paper-on-children-schools-transmission.pdf + https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/935102/sage-65-meeting-covid-19-s0863.pdf
This paper presents evidence for increased transmission occurring among school children when schools are open, particularly in children of secondary school age (high confidence). Multiple data sources show a reduction in transmission in children following schools closing for half-term, and transmission rates increasing again following the post-half-term return to school.
4 January
Sun K, Gu L, Ma L, Duan Y. Atlas of ACE2 gene expression reveals novel insights into transmission of SARS-CoV-2. Heliyon 2020, published 25 December. Full-text: https://doi.org/10.1016/j.heliyon.2020.e05850
Could cats and dogs serve as intermediate hosts of SARS-CoV-2 infection? Here, Kun Sun from Shenzhen, China, describe the conservation of ACE2 and its expression pattern among various mammalian species that are close to human beings. Let’s keep an eye on cats and dogs.
28 December
Birgand G, Pfeiffer-Smadja N, Fournier S, et al. Assessment of Air Contamination by SARS-CoV-2 in Hospital Settings. JAMA Netw Open 2020;3(12):e2033232. Full-text: https://doi.org/10.1001/jamanetworkopen.2020.33232
Controversy remains regarding the level of air contamination from SARS-CoV-2 in hospitals. After reviewing 24 cross-sectional observational studies, the authors report that the air close to and distant from patients with coronavirus disease 2019 was frequently contaminated with SARS-CoV-2 RNA; however, few of these samples contained viable virus. High viral loads were found in toilets and bathrooms, staff areas, and public hallways.
26 December
Aydillo T, Gonzales-Reiche AS, Aslam S, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med 2020, published 24 December. Full-text: https://doi.org/10.1056/NEJMc2031670
Patients with profound immunosuppression may shed viable SARS-CoV-2 for at least 2 months. This is the results of a study by Mini Kamboj, Teresa Aydillo and colleagues from Memorial Sloan Kettering Cancer Center, New York. The authors used cell cultures to detect viable virus in serially collected respiratory samples obtained from 18 recipients of hematopoietic stem cell transplants or chimeric antigen receptor (CAR) T cell therapy and 2 patients with lymphoma.
25 December
Kemp SA, Collier DA, Datir R, et al. Neutralising antibodies drive Spike mediated SARS-CoV-2 evasion. medRxiv 2020, posted 19 December. Full-text: https://doi.org/10.1101/2020.12.05.20241927
In immune-suppressed individuals, treatment with convalescent plasma might lead to the emergence of a mutated SARS-CoV-2 strain. Here, Ravindra Gupta from the Cambridge Institute for Therapeutic Immunology and Infectious Diseases reports the case of a repeated evolutionary response by SARS-CoV-2 against antibody therapy during the course of a persistent and eventually fatal infection in an immunocompromised host. The authors describe a 69-70 deletion in the spike protein which was twice as infectious in a lentivirus model. The 69-70 deletion is also present in the new UK variant strain B.1.1.7.
Read also Kupferschmidt K. Mutant coronavirus in the United Kingdom sets off alarms, but its importance remains unclear. Nature 2020, published 20 December. Full-text: https://www.sciencemag.org/news/2020/12/mutant-coronavirus-united-kingdom-sets-alarms-its-importance-remains-unclear
+
Kupferschmidt K. U.K. variant puts spotlight on immunocompromised patients’ role in the COVID-19 pandemic. Nature 2020, published 23 December. Full-text: https://www.sciencemag.org/news/2020/12/uk-variant-puts-spotlight-immunocompromised-patients-role-covid-19-pandemic
23 December
Eisenstein M. What’s your risk of catching COVID? These tools help you to find out. Nature 2020, published 21 December. Full-text: https://www.nature.com/articles/d41586-020-03637-y
Several apps have been recently launched that claim to predict the chance of infection and illness depending on what people are doing and where they are. In this Technology Feature, Michael Eisenstein takes a closer look.
21 December
European Centre for Disease Prevention and Control. Threat Assessment Brief: Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. ECDC 2020, published 20 December. Full-text: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-rapid-increase-sars-cov-2-variant-united-kingdom
+
European Centre for Disease Prevention and Control. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. ECDC 2020, published 20 December. Full-text: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf
South East England has recently faced a rapid increase in COVID-19 cases. Analysis of viral genome sequence data showed that a large proportion of cases belonged to a new single phylogenetic cluster. The new variant is defined by multiple spike protein mutations (deletion 69-70, deletion 144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H) present as well as mutations in other genomic regions. Preliminary analysis in the UK suggests that this variant is more transmissible than previously circulating variants, with an estimated potential to increase the reproductive number (R) by 0.4 or greater with an estimated increased transmissibility of up to 70%. There is no indication at this point of increased infection severity associated with the new variant.
Sayampanathan AA, Heng CS, Pin PH, et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet December 18, 2020. Full-text: https://doi.org/10.1016/S0140-6736(20)32651-9
Some evidence of a lower infectivity of asymptomatic patients. Andrew Sayampanathan and colleagues looked at 628 people with COVID-19 and 3790 close contacts in Singapore. Overall, 89 (2%) of 3790 close community contacts developed COVID-19 while in quarantine. Binomial regression revealed that when adjusted for age, gender, and serology of index case, the incidence of COVID-19 among close contacts of a symptomatic index case was 3,85 times higher than for close contacts of an asymptomatic index case (95% CI 2.06–7.19; p < 0·0001).
Heinrich F, Meißner K, Langenwalder F, et al. Postmortem stability of SARS-CoV-2 in nasopharyngeal mucosa. Emerg Infect Dis December 16, 2020. Full-text: https://wwwnc.cdc.gov/eid/article/27/1/20-3112_article
Be careful with human corpses: Fabian Heinrich and colleagues from Hamburg found that nasopharyngeal viral RNA stability in 79 corpses showed no time-dependent decrease. Maintained infectivity was supported by virus isolation up to 35 hours post-mortem. There was no correlation between the post-mortem interval (time of death until cooling at 4°C; median 17,8 hours) and the viral RNA loads of corpses. According to the authors, their data indicate potentially high infectivity of human corpses, requiring hazard assessments in professional fields concerned and careful and conscious handling.
19 December
Jones JM, Kracalik I, Rana MM, Nguyen A, Keller BC, Mishkin A, et al. SARS-CoV-2 infections among recent organ recipients, March–May 2020, United States. Emerg Infect Dis 2021. Full-text: https://doi.org/10.3201/eid2702.204046
In March 2020, US transplant centers began to report potential donor-derived SARS-CoV-2 transmission to the Organ Procurement and Transplantation Network (OPTN). For 8 potential donor-derived SARS-CoV-2 transmissions reported to the OPTN during March–May 2020, the available evidence suggest that the most likely source of transmission was community or healthcare exposure, not the organ donor.
13 December
Lemieux JE, Siddle KJ, Shaw BM, et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science 2020, published 10 December. Full-text: https://doi.org/10.1126/science.abe3261
Understanding the role of superspreading events in transmission is critical for prioritizing public health interventions. Here, Jacob Lemieux et al. report the analysis of 772 complete SARS-CoV-2 genomes from early in the Boston area epidemic. They found numerous introductions of the virus, a small number of which led to most cases. Find more about two superspreading events in a skilled nursing facility and at an international business conference and varying transmission dynamics in superspreading events.
11 December
Becker AD, Grantz KH, Hegde ST, et al. Development and dissemination of infectious disease dynamic transmission models during the COVID-19 pandemic: what can we learn from other pathogens and how can we move forward? Lancet Digital Health 2020, published 7 December. Full-text: https://doi.org/10.1016/S2589-7500(20)30268-5
Transmission models offer a systematic way to investigate transmission dynamics and produce predictions that integrate assumptions about biological, behavioral, and epidemiological processes. These models also generate possible trajectories of disease burden, evaluate the effectiveness of intervention strategies, and estimate key transmission variables. In this review, the authors highlight key aspects of the history of infectious disease dynamic models. Learn more about malaria, HIV/AIDS, measles, rubella, foot and mouth disease and Ebola.
8 December
Muller N, Kunze M, Steitz F, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak Related to a Nightclub, Germany, 2020. Emerg Infect Dis. 2020 Dec 2;27(2). PubMed: https://pubmed.gov/33263514. Full-text: https://doi.org/10.3201/eid2702.204443
Club X is located in a basement in Berlin, Germany. The area accessible to guests is 150 m2 with a height of 3 m. Ventilation of the space is ensured by a mechanical air exhaust and supply system and maintenance was performed according to the manufacturer’s instructions. To avoid noise pollution in the surrounding neighborhood, windows are usually closed during events. On February 29, Club X held an event attended by 300 guests and an index patient who had self-reported initial symptoms one day before attending event. Results: 74 reported cases were linked to the outbreak. Staff members were particularly affected (attack rate 56%) and likely caused sustained viral transmission after an event at the club. This outbreak illustrates the potential for superspreader events and supports current club closures.
6 December
Alsved M, Matamis A, Bohlin R, et al. Exhaled respiratory particles during singing and talking. Aerosol Research Letters 2020, published 17 September. Full-text: https://doi.org/10.1080/02786826.2020.1812502
Aerosols are emitted from breathing, talking, coughing and sneezing. Over time, normal breathing can generate more viable viral aerosol than even coughing! In this study, Jacob Löhndal, Malin Alsved and colleagues investigated aerosol and droplet emissions during singing, as compared to talking and breathing. As expected, normal singing generated significantly more aerosol particles than normal talking and loud singing produced more particles than normal singing. (Advice of the Editors: Get vaccinated – and then wait 57 days – before you go back to your weekly choir sessions.)
5 December
Patterson EI, Elia G, Grassi A, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat Commun 11, 6231 (2020). Full-text: https://doi.org/10.1038/s41467-020-20097-0
A pre-print paper we presented on 27 July has now been published in Nat Commun. In the final version, Nicola Decaro and colleagues describe SARS-CoV-2 infection in 919 companion animals in northern Italy at the height of the spring 2020 epidemic. Although no animals tested PCR positive, 3,3% of dogs and 5,8% of cats had measurable SARS-CoV-2 neutralizing antibody titers, with dogs from COVID-19 positive households being significantly more likely to test positive than those from COVID-19 negative households. From their experience, the authors conclude that it is unlikely that infected pets play an active role in SARS-CoV-2 transmission to humans. Only under special circumstances, such as the high animal population densities encountered on infected mink farms, might animal-to-human transmission be more likely.
1 Dezember
Bulfone TC, Malekinejad M, Rutherford, et al. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses, a Systematic Review. J Inf Dis November 29, 2020. Full-text: https://doi.org/10.1093/infdis/jiaa742
According to this review, existing evidence supports the widely held belief that the risk of SARS-CoV-2 transmission is lower outdoors. Moreover, historical evidence gleaned from influenza outbreaks further support the lower risk of transmission outdoors. However, there are significant gaps in our understanding of specific pathways. It is important to note that infections are possible outdoors and the advantage may be overtaken by relaxed mitigation efforts (think of the White House outbreak on September 26).
28 November
Cunningham L, Nicholson PJ, O’Cnnor J, McFadden JP. Cold working environments as an occupational risk factor for COVID-19. Occup Med 2020, published 28 November. Full-text: https://doi.org/10.1093/occmed/kqaa195
Employers and their occupational health and safety professionals should consider work in cold environments to be an independent occupational risk factor for developing COVID-19. Follow Louise Cunningham et al. on their tour in cold environments.
26 November
Scudellari M. How Iceland hammered COVID with science. Nature 2020, published 25 November. Full-text: https://www.nature.com/articles/d41586-020-03284-3
In this semi-interview with Kári Stefánsson, the founder and chief executive of deCODE genetics, Megan Scudellari describes how a private company and health authorities worked hand-in-hand, sharing ideas, data, laboratory space and staff. This collaboration, coupled with Iceland’s diminutive size, has put the country in the enviable position of knowing practically every move the virus has made. The teams have tracked the health of every person who has tested positive for SARS-CoV-2, sequenced the genetic material of each viral isolate and screened more than half of the island’s 368.000 residents for infection. A tiny world laboratory.
van Dorp L, Richard D, Tan CCS, et al. No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2. Nat Commun 11, 5986 (2020). Full-text: https://doi.org/10.1038/s41467-020-19818-2
As yet no evidence for increased transmissibility of SARS-CoV-2. After analyzing 46.723 SARS-CoV-2 genomes isolated from patients worldwide, Lucy van Dorp, François Balloux and colleagues did not identify a single recurrent mutation in this set convincingly associated with increased viral transmission. Instead, recurrent mutations seem to be primarily induced by host immunity through RNA editing mechanisms, and likely tend to be selectively neutral, with no or only negligible effects on virus transmissibility.
25 November
Sun K, Wang W, Gao L, et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 24 Nov 2020: eabe2424. Full-text: https://doi.org/10.1126/science.abe2424
Using detailed information on 1178 SARS-CoV-2 infected individuals along with their 15.648 contacts in Hunan, China, Kaiyuan Sun and colleagues dissected the behavioral and clinical drivers of SARS-CoV-2 transmission. They also evaluated the plausibility of SARS-CoV-2 control through individual and population-based interventions. Of note, 80% of secondary infections traced back to 15% of SARS-CoV-2 primary infections. Transmission risk scales positively with the duration of exposure and the closeness of social interactions and is modulated by demographic and clinical factors. Modeling indicates SARS-CoV-2 control requires the synergistic efforts of case isolation, contact quarantine, and population-level interventions, owing to the specific transmission kinetics of this virus.
22 November
Van Dyke ME, Rogers TM, Pevzner E, et al. Trends in County-Level COVID-19 Incidence in Counties With and Without a Mask Mandate — Kansas, June 1–August 23, 2020. MMWR Morb Mortal Wkly Rep. ePub: 20 November 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6947e2
Masks work! The governor of Kansas issued an executive order requiring wearing masks in public spaces, effective July 3, 2020, which was subject to county authority to opt out. After July 3, COVID-19 incidence decreased in 24 counties with mask mandates but continued to increase in 81 counties without mask mandates. By August 17–23, 2020, the 7-day rolling average COVID-19 incidence had decreased by 6% to 16 cases per 100.000 among mandated counties and increased by 100% to 12 per 100.000 among non-mandated counties.
20 November
Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2020, published 19 November. Full-text: https://doi.org/10.1016/S2666-5247(20)30172-5
After this meta-analysis of 79 studies (5340 individuals) on SARS-CoV-2, the authors report that no study detected live virus beyond day 9 of illness, despite persistently high viral loads. Although SARS-CoV-2 RNA shedding in respiratory and stool samples can be prolonged, duration of viable virus is relatively short-lived. Please communicate this finding to state authorities (for example, in Italy) which require negative RT-PCR tests before allowing citizens who recovered from SARS-CoV-2 infection to go back to normal life.
Heidt A. COVID-19 Diagnostics: How Do Saliva Tests Compare to Swabs? The Scientist 2020, published 9 October. Full-text: https://www.the-scientist.com/news-opinion/covid-19-diagnostics-how-do-saliva-tests-compare-to-swabs–68035
A lay press article going in the same direction. From hospitals and college campuses to remote villages in French Guiana, scientists pit the two approaches against one other.
13 November
The pre-print paper we presented on October 25 has been published in Science: Hou YJ, Chiba S, Halfmann P, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020, published 12 November. Full-text: https://doi.org/10.1126/science.abe8499
This paper is a milestone work: Ralph Baric and colleagues (among the leading labs in the world) provide the possible explanation for the exploding numbers of SARS-CoV-2 infections. Engineering SARS-CoV-2 variants harboring the D614G substitution (the most prevalent SARS-CoV-2 strain circulating globally), they show that the D614G variant replicates more efficiency in primary human proximal airway epithelial cells and is more fit than wildtype virus in competition studies. Infection of human ACE2 transgenic mice and Syrian hamsters with the wildtype or D614G viruses produced similar titers in respiratory tissue and pulmonary disease. However, the D614G variant exhibited significantly faster droplet transmission between hamsters than the WT virus, early after infection. No more doubts that the SARS-CoV2 D614G substitution enhances infectivity, replication fitness, and early transmission.
Liu J, Li Y, Liu L, et al. Infection of human sweat glands by SARS-CoV-2. Cell Discov 6, 84 (2020). Full-text: https://doi.org/10.1038/s41421-020-00229-y
Manli Wang, Jia Liu and colleagues from Wuhan Institute of Virology describe skin autopsy samples from five patients with COVID-19. Immunofluorescence and immunohistochemical analyses detected SARS-CoV-2 spike proteins in three of the five patients. In these cases, the virus resided primarily in the sweat glands and sweat ducts with apparently higher amounts in the former than in the latter; in contrast, the virus was rarely detected in the epidermis or sebaceous glands. The authors conclude that “it is important to further assess the potential risk of viral transmission via perspiration and skin contact.” Editor’s note: This paper will not change my standard protection measures.
12 November
Letizia AG, Ramos I, Obla A, et al. SARS-CoV-2 Transmission among Marine Recruits during Quarantine. N Engl J Med 2020, published 11 November. Full-text: https://doi.org/10.1056/NEJMoa2029717
A two-week quarantine at home is not sufficient to prevent SARS-CoV-2 from entering into a closed college campus. That’s one of the results of a study by Stuart Sealfon, Andrew Letizia and colleagues who investigated SARS-CoV-2 infections among US Marine Corps. Around 2% of recruits who had had negative results for SARS-CoV-2 at the beginning of a supervised quarantine tested positive within two weeks. Most recruits who tested positive were asymptomatic, and no infections were detected through daily symptom monitoring. The author’s short conclusion: “Transmission clusters occur within platoons.”
See also the comment by Michael NL. SARS-CoV-2 in the U.S. Military — Lessons for Civil Society. N Engl J Med 2020, published 11 November. Full-text: https://doi.org/10.1056/NEJMe2032179
Kasper MR, Geibe JR, Sears CL, et al. An outbreak of Covid-19 on an aircraft carrier. N Engl J Med 2020, published 11 November. Full-text: https://doi.org/10.1056/NEJMoa2019375
Do you remember March 30? SARS-CoV-2 is spreading aboard the aircraft carrier USS Theodore Roosevelt. The ship’s commanding officer, Captain Brett Crozier, sends an email to three admirals in his chain of command, recommending that he be given permission to evacuate all non-essential sailors, to quarantine known COVID-19 cases, and sanitize the ship. “We are not at war. Sailors do not need to die,” writes Crozier in his four-page memo. The letter leaks to the media and generates several headlines. Three days later, 2 April, Captain Crozier is sacked.
You can now read the final report of the outbreak. A total of 1271 crew members (26.6% of the crew) tested positive for SARS-CoV-2. Among the crew members with laboratory-confirmed infection, 76.9% (978 of 1271) had no symptoms at the time that they tested positive and 55.0% had symptoms develop some time during the clinical course. Among the 1331 crew members with suspected or confirmed COVID-19, 23 (1.7%) were hospitalized, 4 (0.3%) received intensive care, and 1 died. Crew members who worked in confined spaces appeared more likely to become infected. The conclusion: transmission of SARS-CoV-2 is facilitated by close-quarters conditions and by asymptomatic and pre-symptomatic infected crew members.
Nissen K, Krambrich J, Akaberi D, et al. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci Rep 10, 19589 (2020). Full-text: https://doi.org/10.1038/s41598-020-76442-2
Detection of coronavirus RNA, including SARS-CoV-2, in hospital and other ventilation systems has been reported. Here, Erik Salaneck, Karolina Nissen and colleagues present further evidence for SARS-CoV-2’s ability to disperse from patients to ward vent openings. They detected viral RNA in ventilation exhaust filters located at least 50 m from patient room vent openings. Although the authors did not isolate infectious virus, they suggest that there may be a risk for airborne dissemination and transmission, especially at much closer distances to contagious persons in confined spaces, both in and outside hospital environments.
11 November
Chang S, Pierson E, Koh PW. Mobility network models of COVID-19 explain inequities and inform reopening. Nature (2020). Full-text: https://doi.org/10.1038/s41586-020-2923-3
A small minority of “superspreader” events account for a large majority of infections. What we have known for a long time is now being confirmed by a mobility network model which mapped the hourly movements of 98 million people from neighborhoods to points of interest (POIs) such as restaurants and religious establishments. Jure Leskovec, Serina Chang and colleagues, connected 57,000 neighborhoods to 553,000 POIs and found that restricting maximum occupancy at each POI would be more effective than uniformly reducing mobility. Their model also correctly predicted higher infection rates among disadvantaged racial and socioeconomic groups: disadvantaged groups cannot reduce mobility as sharply as other groups and the POIs they visit are more crowded.
See also the comment by Ma KC, Lipsitch M. Big data and simple models used to track the spread of COVID-19 in cities. Nature 2020, published 10 November. Full-text: https://www.nature.com/articles/d41586-020-02964-4
See also Cyranoski D. How to stop restaurants from driving COVID infections. Nature 2020, published 10 November. Full-text: https://www.nature.com/articles/d41586-020-03140-4 | US mobile data suggests restaurants, gyms and cafes can be COVID hotspots — and reveals strategies for limiting spread.
5 November
Tufekci Z. We Need to Talk About Ventilation. The Atlantic 2020, published 30 July. Full-text: https://www.theatlantic.com/health/archive/2020/07/why-arent-we-talking-more-about-airborne-transmission/614737/
Published more than three months ago but still instructive.
Harvey AP, Fuhrmeister ER, Cantrell M, et al. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. medRxiv 2020, posted 1 November. Full-text: https://doi.org/10.1101/2020.10.27.20220905
The estimated risk of infection from touching a contaminated surface was less than 5 in 10,000 in a study by Amy Pickering, Abigail Harvey and colleagues. From April to June 2020, they repeatedly sampled 33 surfaces in public places like liquor and grocery stores, banks, gas stations, laundromats, restaurants and on metro doors and crosswalk buttons. Twenty-nine of 348 (8.3 %) surface samples were positive for SARS-CoV-2. The authors suggest that fomites might play only a minimal role in SARS-CoV-2 community transmission.
A second, not less important point: The weekly percentage of positive samples in one postal district peaked about 7 days before a spike in new SARS-CoV-2 cases. Surveillance on high-touch surfaces might therefore provide precious early warning clues.
3 November
Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis 2020, published 2 November. Full-text: https://doi.org/10.1016/S1473-3099(20)30833-1
Excellent retrospective cohort study by Vernon Lee, Oon Tek Ng and colleagues Between Jan 23 and April 3, 2020, the authors identified 7,518 close contacts (1779 [23%] household contacts, 2231 [30%] work contacts, and 3508 [47%] social contacts) linked to 1114 PCR-confirmed index cases. The secondary clinical attack rate:
Household contacts: 5.9% (95% CI 4.9–7.1).
Particular risk factors:
- Sharing a bedroom (multivariable odds ratio [OR] 5.38)
- Being spoken to by an index case for 30 min or longer (7.86)
Non-household contact: Work contacts, 1.3% (0.9–1.9); social contacts, 1.3% (1.0–1.7).
Particular risk factors:
- Exposure to more than one case (3.92)
- Being spoken to by an index case for 30 min or longer (2.67)
- Sharing a vehicle with an index case (3.07).
Among both household and non-household contacts, indirect contact, meal sharing, and lavatory co-usage were not independently associated with SARS-CoV-2 transmission.
1 November
Wang Y, Xu G, Huang YW. Modeling the load of SARS-CoV-2 virus in human expelled particles during coughing and speaking. PLoS One. 2020 Oct 30;15(10):e0241539. PubMed: https://pubmed.gov/33125421 . Full-text: https://doi.org/10.1371/journal.pone.0241539
The authors investigated the dependence of airborne viral load on the size distributions of the human expelled particles from coughing and speaking. Of note, differentiating “aerosols” and “droplets” using a specific size, e.g., 5 μm, did not reflect the actual evolution of virus-containing particles over time and space, because a large number of particles above 5 μm remained airborne after an extended period of time. Simulation showed that after ten seconds of a cough, although most evaporated particles are larger than 5 μm, 59.5% of the original virus-containing particles were still able to remain airborne. Simulation also showed that wearing a mask can effectively reduce the spread of the viruses. The results challenge the false dichotomy of using aerosols and droplets to separate the modes of disease transmission.
31 October
Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 Infections in Households — Tennessee and Wisconsin, April–September 2020. MMWR Morb Mortal Wkly Rep. ePub: 30 October 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6944e1
Important study (because prospective). After enrollment, 101 index patients and 191 household members were trained remotely by study staff members to complete symptom diaries and obtain self-collected specimens, nasal swabs only or nasal swabs and saliva samples, daily for 14 days. In this preliminary analysis of the first 7 days, transmission of SARS-CoV-2 among household members was common, and secondary infection rates were higher (35%) than have been previously reported. Secondary infections occurred rapidly and were high across all racial/ethnic groups or rage groups. The authors conclude that persons who suspect that they might have COVID-19 should isolate, stay at home, and use a separate bedroom and bathroom if feasible. Isolation should begin before seeking testing and before test results become available. All household members, including the index patient, should start wearing a mask in the home, particularly in shared spaces where appropriate distancing is not possible. Close household contacts of the index patient should also self-quarantine, to the extent possible, particularly staying away from those at higher risk of getting severe COVID-19.
Shah ASV, Wood R, Gribben C. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ October 28, 2020;371:m3582. Full-text: http://dx.doi.org/10.1136/bmj.m3582
This large cohort from Scotland comprising 158,445 healthcare workers (HCW) and 229,905 household members revealed that HCW and their households contributed a sixth of all COVID-19 cases admitted to hospital. Patient facing HCW and their household members had threefold and twofold increased risks of admission with COVID-19. However, the absolute risk of admission was low overall, being 0.20% (181/90733), 0.07% (23/32615), and 0.11% (39/35097) in patient facing, non-patient facing, and undetermined HCW.
Goldstein E, Lipsitch M, Cevik M, et al. On the effect of age on the transmission of SARS-CoV-2 in households, schools and the community. J Inf Dis 29 October 2020, jiaa691. Full-text: https://doi.org/10.1093/infdis/jiaa691
Literature review of all papers published before October 5, 2020. Compared to younger/middle aged adults, susceptibility to infection for children aged less than 10 years of age seems to be significantly lower, while estimated susceptibility to infection in adults aged over 60 years is higher. There is some evidence that given limited control measures, SARS-CoV-2 may spread robustly in secondary/high schools, and to a lesser degree in primary schools, with class size possibly affecting that spread. There is also evidence of more limited spread in schools when some mitigation measures are implemented. Several potential biases that may affect these studies are discussed.
28 October
Lee EC, Wada NI, Grabowski MK, Gurley ES, Lessler J. The engines of SARS-CoV-2 spread. Science. 2020 Oct 23;370(6515):406-407. PubMed: https://pubmed.gov/33093098. Full-text: https://doi.org/10.1126/science.abd8755
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mostly transmitted within households and household-like settings. A decreasing proportion of transmission events take place at increasing spatial scales, but these events become more critical for sustaining the pandemic. Discover the fundamental engines that drive the SARS-CoV-2 pandemic in this Science Perspective.
Teran RA, Ghinai I, Gretsch S, et al. COVID-19 Outbreak Among a University’s Men’s and Women’s Soccer Teams — Chicago, Illinois, July–August 2020. MMWR Morb Mortal Wkly Rep. ePub: 27 October 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6943e5
Investigation of 17 COVID-19 cases among a university’s men’s and women’s soccer team identified numerous social gatherings as possible transmission events. Minimal mask use and social distancing resulted in rapid spread among students who live, practice, and socialize together. Nothing truly new – we should probably stop reporting on SARS-CoV-2 transmission during social gatherings.
25 October
Hou YJ, Chiba S, Halfmann P, et al. SARS-CoV-2 D614G Variant Exhibits Enhanced Replication ex vivo and Earlier Transmission in vivo. bioRxiv. 2020 Sep 29:2020.09.28.317685. PubMed: https://pubmed.gov/33024969. Full-text: https://doi.org/10.1101/2020.09.28.317685.
This is a pre-print and not a peer reviewed paper. However, this milestone work from Ralph Baric and colleagues (among the leading labs in the world) probably provides the explanation for the exploding numbers. The D614G substitution in the S protein is now the most prevalent SARS-CoV-2 strain circulating globally. Engineering SARS-CoV-2 variants harboring the D614G substitution, the Baric group shows that the D614G variant replicates more efficiently in primary human proximal airway epithelial cells and is more fit than wildtype virus in competition studies. Infection of human ACE2 transgenic mice and Syrian hamsters with wildtype (WT) or D614G viruses produced similar titers in respiratory tissue and pulmonary disease. However, the D614G variant exhibited significantly faster droplet transmission between hamsters than the WT virus early after infection. No more doubts that the SARS-CoV2 D614G substitution enhances infectivity, replication fitness, and early transmission. Fantastic work, but probably the worst news of the last few months.
Murphy N, Boland M, Bambury N, et al. A large national outbreak of COVID-19 linked to air travel, Ireland, summer 2020. Euro Surveill 2020;25(42):pii=2001624. Full-text: https://doi.org/10.2807/1560-7917.ES.2020.25.42.2001624
Honestly, if you had seen this selection of seats, wouldn’t you have felt safe? This was a 7.5 h flight to Ireland, with a passenger occupancy of 17% (49/283 seats). Astonishingly, the flight-associated attack rate was 9,8–17,8%, leading to 13 cases (in-flight transmission was proven by 99% homology across the virus genome in five cases travelling from three different continents). A mask was worn during the flight by nine cases, not worn by one (a child), and unknown for three. Spread to 46 non-flight cases occurred country-wide. No need to explain why this is bad news.
Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a Correctional Facility Employee Following Multiple Brief Exposures to Persons with COVID-19 — Vermont, July–August 2020. MMWR Morb Mortal Wkly Rep. ePub: 21 October 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6943e1
On August 11, 2020, a young male correctional officer was found to be infected. Some days earlier, he had multiple brief encounters with six infected incarcerated or detained persons (IDPs). Other infection routes were ruled out. Why is this of interest? Because detailed video surveillance footage was available! It showed that the officer had no close contact (being within 6 feet of infectious persons for ≥ 15 consecutive minutes) but numerous brief (approximately 1-minute) encounters. During his 8-hour shift, he was within 6 feet of an infectious IDP an estimated 22 times while the cell door was open, for an estimated 17 total minutes of cumulative exposure. IDPs wore microfiber cloth masks during most interactions with the correctional officer that occurred outside a cell; however, during several encounters IDPs did not wear masks. During all interactions, the correctional officer wore a microfiber cloth mask, gown, and eye protection (goggles). He also wore gloves during most interactions. This is bad news because we can’t rely on definitions of “close contacts”.
24 October
Poole S, Brendish NJ, Tanner AR, et al. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Resp Med October 22, 2020. Full-text: https://doi.org/10.1016/S2213-2600(20)30502-6
Stephen Poole and colleagues from Southampton, UK, compared the rate of respiratory virus detection in 2020 with the same period in 2019. Among 3898 adult patients who were tested between March and September, they observed a drop in the rate of detection of all respiratory viruses including rhinovirus following the nationwide lockdown. Around 2 weeks after the concurrent re-opening of state primary and secondary schools in early September, there was a sharp increase in the number of detections similar to that seen in 2019. Two conclusions: 1. children are a major reservoir for rhinovirus infection, and a key driver of transmission to adults. 2. Current physical distancing measures adopted by schools do not effectively prevent rhinovirus transmission.
20 October
Richmond CS, Sabin AP, Jobe DA, et al. SARS-CoV-2 sequencing reveals rapid transmission from college student clusters resulting in morbidity and deaths in vulnerable populations. medRxiv 2020, posted 14 October. Full-text: https://doi.org/10.1101/2020.10.12.20210294
The title says it all: a substantial SARS-CoV-2 outbreak coincided with the return to in-person instruction at three local academic institutions. From 111 sequenced genomes the authors identified rapid transmission of the virus into more vulnerable populations. One of the variants made its way into two care homes, infecting 8 residents. Two died. MedRxiv article – not yet peer reviewed.
19 October
Chau NVV, Hong NTT, Ngoc NM, et al. Superspreading event of SARS-CoV-2 infection at a bar, Ho Chi Minh City, Vietnam. Emerg Infect Dis. 2021 Jan. Full-text: https://doi.org/10.3201/eid2701.203480
From 10:00 PM on March 14 until 2:30 AM of the next day, a 43-year old man participated in a St. Patrick’s Day celebration at a bar in Ho Chi Minh City. The bar had 2 indoor areas for clients, a 300 m2 area downstairs and an 50 m2 area upstairs, with no mechanical ventilation. During opening hours, the left and right entrances were typically kept closed to facilitate cooling with air conditioners that recycle indoor air; the middle entrance was kept open. The bar also has naturally ventilated outdoor spaces. Results: 12 additional cases at the bar (and more, via contacts of people infected there).
18 October
Sugano N, Ando W, Fukushima W. Cluster of Severe Acute Respiratory Syndrome Coronavirus 2 Infections Linked to Music Clubs in Osaka, Japan. J Infect Dis. 2020 Oct 13;222(10):1635-1640. PubMed: https://pubmed.gov/32840606. Full-text: https://doi.org/10.1093/infdis/jiaa542
Detailed contact tracing in Osaka, Japan: The data of 108 cases comprising a cluster were linked to 4 music clubs. In total, 51 cases attended a live music club only once and all index cases for secondary transmission were asymptomatic at the time of contact with other people. Substantial exposure occurred within a few hours. Asymptomatically infected persons can transmit the virus as soon as 2 days after infection. Bad news for music clubs.
Okarska-Napierała M, Mańdziuk J, Kuchar E. SARS-CoV-2 cluster in nursery, Poland. Emerg Infect Dis. 2021 Jan. Full-text: https://doi.org/10.3201/eid2701.203849
Several reports have implied little to no SARS-CoV-2 transmission among children and from children to adults. In this cluster that emerged in a single nursery in Poland within 2 weeks of its reopening, a high infection attack rate among children was found. The cluster involved a total of 29 persons; 8 were children attending the nursery, and 12 were the children’s family members who did not enter the facility. The high attack rates could be explained by prolonged close contact between very young children, who are less able to adjust to control measures. However, these observations question the role of young children in driving the COVID-19 pandemic.
17 October
Atrubin D, Wiese M, Bohinc B. An Outbreak of COVID-19 Associated with a Recreational Hockey Game — Florida, June 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6941a4
On June 16, 2020, a recreational ice hockey game was played between two teams, each consisting of 11 players (typically six on the ice and five on the bench at any given time). The players were men aged 19–53 years. During the 5 days after the game, 15 persons (14 of the 22 players and a rink staff member) experienced signs and symptoms compatible with coronavirus disease 2019 (COVID-19).
Do you remember our September 12 Top 10? In an unintentional experiment, the German national team of amateur boxers proved that you can achieve a 100% transmission rate in a small group within days. In a training camp, some of the 18 athletes and 7 coaches and supervisors had cold symptoms four days prior. All 25 persons tested positive for SARS-CoV-2. If you read German, read Anonymous. Deutsche Box-Olympiamannschaft mit Coronavirus infiziert. Die Zeit 2020, published 12 September. Full-text: https://www.zeit.de/sport/2020-09/trainingslager-oesterreich-deutsche-box-olympiamannschaft-coronavirus-infektion-quarantaene
10 October
Wilson RF, Sharma AJ, Schluechtermann S, et al. Factors Influencing Risk for COVID-19 Exposure Among Young Adults Aged 18–23 Years — Winnebago County, Wisconsin, March–July 2020. MMWR Morb Mortal Wkly Rep. ePub: 9 October 2020. DOI: http://dx.doi.org/10.15585/mmwr.mm6941e2
Still in the US: What are the drivers of behaviors that might influence risk for COVID-19 exposure among young adults? In a remote US county, these were low severity of disease outcome; peer pressure; and exposure to misinformation, conflicting messages, or opposing views regarding masks. A scientifically inspired national prevention policy would have been helpful.
8 October
Wang L, Didelot X, Yang J, et al. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phase. Nat Commun 11, 5006 (2020). Full-text: https://doi.org/10.1038/s41467-020-18836-4
Super-spreading events are an important phenomenon in the transmission of many diseases (such as SARS-CoV-1, MERS-CoV, Ebola virus, etc.), in which certain individuals infect a disproportionately large number of people. Here Yuhai Bi, Liang Wang and colleagues show that super-spreading events played an important role in the early stage of the COVID-19 outbreak. They estimated the dispersion parameter to be 0.23 (95% CI: 0.13–0.39). (What is the dispersion parameter? Check this FT article: To beat Covid-19, find today’s superspreading ‘Typhoid Marys’)
7 October
Prather KA, Marr LC, Schooley RT, et al. Airborne transmission of SARS-CoV-2. Science 05 Oct 2020: eabf0521. Full-text: https://doi.org/10.1126/science.abf0521
According to Kimberly Prather and colleagues, we must clarify the terminology to distinguish between aerosols and droplets using a size threshold of 100 μm, not the historical 5 μm. This size more effectively separates their aerodynamic behavior, ability to be inhaled, and efficacy of interventions. Viruses in droplets (larger than 100 μm) typically fall to the ground in seconds within 2 m of the source and can be sprayed like tiny cannonballs onto nearby individuals.
6 October
Schwartz NG, Moorman AC, Makaretz A, et al. Adolescent with COVID-19 as the Source of an Outbreak at a 3-Week Family Gathering — Four States, June–July 2020. MMWR Morb Mortal Wkly Rep. ePub: 5 October 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6940e2
Children can serve as the source for COVID-19 outbreaks, even when their symptoms are mild. In this outbreak that occurred during a 3-week family gathering of five households, an adolescent aged 13 years was the suspected primary patient. Among the 14 persons who stayed in the same house, 12 experienced symptoms. Of note, none of the additional six family members who maintained outdoor physical distance without face masks during two longer visits (10 and 3 hours) to the family gathering developed symptoms.
3 October
Tufekci Z. This Overlooked Variable Is the Key to the Pandemic. The Atlantic 2020, published 30 September. Full-text: https://www.theatlantic.com/health/archive/2020/09/k-overlooked-variable-driving-pandemic/616548/
Even non-scientists have heard about R0 (pronounced as “r-naught”)—the basic reproductive number of a pathogen, a measure of its contagiousness on average. But even some scientists may have not yet encountered k, the measure of its dispersion. If you haven’t done it before, do it now: explore k. It’s simply a way of asking whether a virus spreads in a steady manner or in big bursts, whereby one person infects many, all at once.
Gebrekidan S, Bennhold K, Apuzzo M, Kirkpatrick DD. Ski, Party, Seed a Pandemic: The Travel Rules That Let Covid-19 Take Flight. The New York Times 2020 published 1 October. Full-text: https://www.nytimes.com/2020/09/30/world/europe/ski-party-pandemic-travel-coronavirus.html
ISCHGL, Austria — They came from across the world to ski in the most famous resorts of the Austrian alps…
2 October
Freedman DO, Wilder-Smith A. In-flight Transmission of SARS-CoV-2: a review of the attack rates and available data on the efficacy of face masks. Journal of Travel Medicine September 25. Full-text: https://doi.org/10.1093/jtm/taaa178.
Review of outbreaks during flights. According to the authors, the absence of large numbers of confirmed and published in-flight transmissions of SARS-CoV is encouraging but not definitive evidence that fliers are safe. At present, based on circumstantial data, strict use of masks appears to be protective. Structured prospective studies to quantitate transmission risk on flight with rigid masking protocols are now most pressing.
30 September
Mondelli MU, Colaneri M, Seminari E, et al. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect Dis September 29, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30678-2
Some arguments that environmental contamination leading to SARS-CoV-2 transmission is unlikely to occur in real-life conditions, provided that standard cleaning procedures and precautions are enforced. The chance of transmission through inanimate surfaces is likely less frequent than hitherto recognized.
29 September
Khanh NC, Thai PQ, Quach H-L, Thi NA-H, Dinh PC, Duong TN, et al. Transmission of severe acute respiratory syndrome coronavirus 2 during long flight. Emerg Infect Dis. 2020 Nov [date cited]. Original Publication Date: September 18, 2020. Full-text: https://doi.org/10.3201/eid2611.203299
If you don’t wear a mask, business class does not protect you from infection: Nguyen Cong Khanh, Pham Quang Thai and colleagues report on a cluster among passengers on a 10-hour commercial flight from London to Hanoi on March 2 (at that time, the use of face masks was not mandatory on airplanes or at airports). Affected persons were passengers, crew, and their close contacts. The authors traced 217 passengers and crew to their final destinations and interviewed, tested, and quarantined them. Among the 16 persons in whom SARS-CoV-2 infection was detected, 12 (75%) were passengers seated in business class along with the only symptomatic person (attack rate 62%). Seating proximity was strongly associated with increased infection risk (risk ratio 7.3, 95% CI 1.2–46.2).
27 September
Asadi S, Cappa CD, Barreda S, et al. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep 10, 15665 (2020). Full-text: https://doi.org/10.1038/s41598-020-72798-7
Masks work with super-emittors! William D. Ristenpart, Sima Asadi and colleagues measured outward emissions of micron-scale aerosol particles by healthy humans performing various expiratory activities while wearing different types of medical-grade or homemade masks. Both surgical masks and unvented KN95 respirators reduced the outward particle emission rates by 90% and 74% on average during speaking and coughing. These masks similarly decreased the outward particle emission of a coughing super-emitter, who for unclear reasons emitted up to two orders of magnitude more expiratory particles via coughing than average. An interesting collateral finding: people speak more loudly, but do not cough more loudly, when wearing a mask.
26 September
An outbreak in Sallent (72 km from Barcelona) with 30 SARS-CoV-2-infected people demonstrates the risk posed by choirs and karaoke in poorly ventilated places. See the video: https://www.youtube.com/watch?v=tuQC-NTLE54. Do not sing and jump around in enclosed spaces!
20 September
Khanh NC, Thai PQ, Quach H-L, Thi NA-H, Dinh PC, Duong TN, et al. Transmission of severe acute respiratory syndrome coronavirus 2 during long flight. Emerg Infect Dis 2020, published 18 September. Full-text: https://doi.org/10.3201/eid2611.203299
The authors report a cluster of cases among passengers on VN54 (Vietnam Airlines), a 10-hour commercial flight from London to Hanoi on March 2, 2020. Among the 16 persons in whom SARS-CoV-2 infection was detected, 12 (75%) were passengers seated in business class along with the only symptomatic person (attack rate 62%). The authors find that blocking middle seats, currently recommended by the airline industry, may in theory prevent some in-flight transmission events but seems to be insufficient to prevent superspreading events. They conclude that the risk for on-board transmission of SARS-CoV-2 during long flights is real and has the potential to cause COVID-19 clusters of substantial size, even in business class–like settings with spacious seating arrangements well beyond the established distance used to define close contact on airplanes.
(Note that at the time, March 2, the use of face masks was not mandatory on airplanes or at airports, and there was no social distancing on the aircraft.)
18 September
Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann Intern Med 2020, published 17 September. Full-text: https://doi.org/10.7326/M20-5008
Eric Meyerowitz et al. present a comprehensive review of the evidence of human SARS-CoV-2 transmission. Their key points:
- Respiratory transmission is the dominant mode of transmission.
- Vertical transmission occurs rarely; transplacental transmission has been documented.
- Cats and ferrets can be infected and transmit to each other, but there are no reported cases to date of transmission to humans; minks transmit to each other and to humans.
- Direct contact and fomite transmission are presumed but are likely only an unusual mode of transmission.
- Although live virus has been isolated from saliva and stool and viral RNA has been isolated from semen and blood donations, there are no reported cases of SARS-CoV-2 transmission via fecal–oral, sexual, or bloodborne routes. To date, there is 1 cluster of possible fecal–respiratory transmission.
17 September
Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med (2020). Full-text: https://doi.org/10.1038/s41591-020-1092-0
Dillon Adam, Peng Wu and colleagues identified 4–7 superspreading events (SSEs) across 51 clusters (n = 309 cases) and estimate that 19% (95% confidence interval, 15–24%) of cases seeded 80% of all local transmissions. After controlling for age, transmission in social settings was associated with more secondary cases than households when controlling for age. Social settings are likely to become major battle grounds of coming SARS-CoV-2 waves.
16 September
Milani GP, Bottino I, Rocchi A, et al. Frequency of Children vs Adults Carrying Severe Acute Respiratory Syndrome Coronavirus 2 Asymptomatically. JAMA Pediatr. Published online September 14, 2020. Full-text: https://doi.org/10.1001/jamapediatrics.2020.3595
Early reports suggested that children, often asymptomatic, might be facilitators of SARS-CoV-2 transmission and amplify local outbreaks. Here, Carlo Agostini, Gregorio Milani and colleagues conducted a study among individuals hospitalized in Milan. About 1% of children and 9% of adults without any symptoms or signs of SARS-CoV-2 infection tested positive for SARS-CoV-2. The authors conclude that their data do not support the hypothesis that children are at higher risk of carrying SARS-CoV-2 asymptomatically than adults. Attention: a retrospective analysis.
15 September
Luo K, Lei Z, Hai Z, et al. Transmission of SARS-CoV-2 in Public Transportation Vehicles: A Case Study in Hunan Province, China. Open Forum Infectious Diseases 13 September 2020, ofaa430. Full-text: https://doi.org/10.1093/ofid/ofaa430
Transmission in a bus. The tour coach was 11.3 meters long and 2.5 meters wide with 49 seats, fully occupied with all windows closed and the ventilation system on during the 2.5-hour trip. Among the 49 passengers (including the driver) who shared the ride with the index person, eight tested positive and eight developed symptoms. The index person sat in the second-to-last row, and the infected passengers were distributed over the middle and rear rows.
13 September
Bax A, Bax CE, Stadnytskyi V, Anfinrud P. SARS-CoV-2 transmission via speech-generated respiratory droplets. Lancet Inf Dis September 11, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30726-X
Spit happens. This group published the impressive NEJM video, visualizing speech-generated oral fluid droplets and suggesting that normal speaking might be an important mode of transmission. Here, the four authors vigorously resist the criticism of other authors who argued that the video experiments were unrealistic. They also provide nice new videos showing speech droplets emitted by four people, when speaking the phrase “spit happens” with the face positioned about 10–15 cm behind a thin sheet of intense green laser light.
Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. N Engl J Med. 2020 May 21;382(21):2061-2063. PubMed: https://pubmed.gov/32294341. Full-text: https://doi.org/10.1056/NEJMc2007800
New video: https://www.youtube.com/watch?v=ooVjNth4ut8
12 September
If you read German, read Anonymous. Deutsche Box-Olympiamannschaft mit Coronavirus infiziert. Die Zeit 2020, published 12 September. Full-text: https://www.zeit.de/sport/2020-09/trainingslager-oesterreich-deutsche-box-olympiamannschaft-coronavirus-infektion-quarantaene
In an unintentional experiment, the German national team of amateur boxers has proved that you can achieve a 100% transmission rate in a small group within days. In a training camp, some of the 18 athletes and 7 coaches and supervisors had cold symptoms four days ago. Now all 25 persons have tested positive for SARS-CoV-2. So far, no serious cases.
Ehrhardt J , Ekinci A , Krehl H , et al. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germany. Euro Surveill. 2020;25(36). Full-text: https://doi.org/10.2807/1560-7917.ES.2020.25.36.2001587
After post-lockdown reopening of schools and childcare facilities in May 2020 in Baden-Württemberg, Germany, child-to-child transmission appeared uncommon. Stefan Brockmann, Jonas Ehrhardt and colleagues anticipate that, with face mask use and frequent ventilation of rooms, transmission rates in schools/childcare facilities could remain low, even if class group sizes were increased.
Lopez AS, Hill M, Antezano J, et al. Transmission Dynamics of COVID-19 Outbreaks Associated with Child Care Facilities — Salt Lake City, Utah, April–July 2020. MMWR Morb Mortal Wkly Rep. ePub: 11 September 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6937e3
Cuc Tran, Adriana Lopez and colleagues describe 12 children who acquired SARS-CoV-2 infection in child-care facilities. All had mild or no symptoms. They transmitted the virus to at least 12 (26%) of 46 non-facility contacts. The authors conclude that testing children who might not have symptoms could improve control of transmission from child-care attendees to family members.
Fisher KA, Tenforde MW, Feldstein LR, et al. Community and Close Contact Exposures Associated with COVID-19 Among Symptomatic Adults ≥18 Years in 11 Outpatient Health Care Facilities — United States, July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1258–1264. Full-text: http://dx.doi.org/10.15585/mmwr.mm6936a5
Eating and drinking and socializing? Everything may well return to normal in about two years. In the meantime, note that adults with a positive SARS-CoV-2 test result were found to be twice as likely to have had dinner at a restaurant than those with negative test results. Kiva Fisher and colleagues conclude that eating and drinking on-site at locations that offer such options might be important risk factors associated with SARS-CoV-2 infection. Bars and restaurants are in for a rough autumn and winter season.
11 September
Bedford T, Greninger AL, Roychoudhury P, et al. Cryptic transmission of SARS-CoV-2 in Washington state. Science 2020, published 10 September. Full-text: https://doi.org/10.1126/science.abc0523
Trevor Bedford et al. analyzed 453 SARS-CoV-2 genomes collected between 20 February and 15 March 2020 from infected patients in Washington State, US. The result: most SARS-CoV-2 infections derived from a single introduction in late January or early February 2020 which subsequently spread locally before active community surveillance was implemented. These results, again, highlight the critical need for widespread surveillance for community transmission of SARS-CoV-2.
4 September
Shen Y, Li C, Dong H. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Intern Med, September 1, 2020. Full-text: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2770172
If you take the bus, choose seats near a window (and open it). On January 19, 2020, 68 individuals (including the source patient) took a bus on a 100-minute round trip to attend a worship event. In total, 24 (35%) received a diagnosis of COVID-19 after the event. The authors were able to identify seats for each passenger and divided bus seats into high-risk and low-risk zones. Passengers in the high-risk zones had moderately but non-significantly higher risk of getting COVID-19 than those in the low-risk zones. On the 3-seat side of the bus, except for the passenger sitting next to the index patient, none of the passengers sitting in seats close to the bus window developed infection. In addition, the driver and passengers sitting close to the bus door also did not develop infection, and only 1 passenger sitting by an operable window developed infection. The absence of a significantly increased risk in the part of the bus closer to the index case suggested that airborne spread of the virus may at least partially explain the markedly high attack rate observed.
2 September
Kang M, Wi J, Yuan J, et al. Probable Evidence of Fecal Aerosol Transmission of SARS-CoV-2 in a High-Rise Building. Ann Intern Med 2020, published 1 September. Full-text: https://doi.org/10.7326/M20-0928
Nanshan Zhong, Min Kang and colleagues report 9 infected patients in 3 families. While the first family had a history of travel to the coronavirus disease 2019 (COVID-19) epicenter Wuhan, the other 2 families had no travel history and a later onset of symptoms. The families lived in 3 vertically aligned flats connected by drainage pipes in the master bathrooms. The authors suggest that virus-containing fecal aerosols may have been produced in the associated vertical stack during toilet flushing after use by the index patients. This report reminds us of a SARS-1 outbreak in March 2003 among residents of Amoy Gardens, Hong Kong, with a total of 320 SARS cases in less than three weeks (see www.SARSReference.com, page 65).
See also the comment by Michael Gormley [Gormley M. SARS-CoV-2: The Growing Case for Potential Transmission in a Building via Wastewater Plumbing Systems. Ann Intern Med 2020, published 1 September. Full-text: https://doi.org/10.7326/M20-6134] concludes that that wastewater plumbing systems, particularly those in high-rise buildings, deserve closer investigation, both immediately in the context of SARS-CoV-2 and in the long term, because they may be a reservoir for other harmful pathogens.
Deng W, Bao L, Gao H, et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 11, 4400 (2020). Full-text: https://doi.org/10.1038/s41467-020-18149-6
If you are exploring extra-respiratory routes of SARS-CoV-2 transmission, read the article by Chuan Qin, Wei Deng and colleagues. The authors inoculated five rhesus macaques with SARS-CoV-2 conjunctivally, intratracheally, and intragastrically. The conjunctivally infected animal had a higher viral load in the nasolacrimal system than the intratracheally infected animal but also showed mild interstitial pneumonia, suggesting distinct viral distributions.
27 August
Simha PP, Rao PSM. Universal trends in human cough airflows at large distances featured. Physics of Fluids 32, 081905 (2020). Published 25 August. Full-text: https://doi.org/10.1063/5.0021666
Fine droplets can pass through layers of masks and are carried away by the exhaled airflow unlike larger droplets that settle down due to gravity. Now Padmanabha Prasanna Simha and Prasanna Simha Mohan Rao visualize the flow fields of coughs under various mouth covering scenarios. The results:
- N95 masks are the most effective at reducing the horizontal spread of a cough (spread: 0.1 and 0.25 meters).
- A simple disposable mask can reduce the spread to 0.5 meters, while an uncovered cough can travel up to 3 meters.
- Coughing into the elbow? Not very effective! Unless covered by a sleeve, a bare arm cannot form the proper seal against the nose necessary to obstruct airflow and a cough is able to leak through any openings and propagate in many directions.
Garigliany M, Van Laere AS, Clercx C, et al. SARS-CoV-2 Natural Transmission from Human to Cat, Belgium, March 2020. Emerg Infect Dis. 2020 Aug 12;26(12). PubMed: https://pubmed.gov/32788033. Full-text: https://doi.org/10.3201/eid2612.202223
And another human-to-cat transmission of SARS-CoV-2.
26 August
Rhee C, Kanjilal S, Baker M, et al. Duration of SARS-CoV-2 Infectivity: When is it Safe to Discontinue Isolation? Clinical Infectious Diseases, 25 August 2020, ciaa1249. Full-text: https://doi.org/10.1093/cid/ciaa1249
Persistently positive RT-PCRs generally do not reflect replication-competent virus. SARS-CoV-2 infectivity rapidly decreases thereafter to near-zero after about 10 days in mild-to-moderately-ill patients and 15 days in severely-to-critically-ill and immunocompromised patients. This review summarizes evidence-to-date on the duration of infectivity of SARS-CoV-2.
Singanayagam A, Patel M, Charlett A. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32). Full-text: https://doi.org/10.2807/1560-7917.ES.2020.25.32.2001483
More on “viral load” and infectivity. Virus culture was attempted from 324 samples (from 253 cases) that tested positive for SARS-CoV-2 by RT-PCR. RT-PCR cycle threshold (Ct) values correlated strongly with cultivable virus. Probability of culturing virus declined to 8% in samples with Ct > 35 and to 6% (95% CI: 0.9–31.2%) 10 days after onset; it was similar in asymptomatic and symptomatic persons.
Lesho E, Reno L, Newhart D, et al. Temporal, Spatial, and Epidemiologic Relationships of SARS-CoV-2 Gene Cycle Thresholds: A Pragmatic Ambi-directional Observation. Clinical Infectious Diseases, 25 August 2020, ciaa1248. Full-text: https://doi.org/10.1093/cid/ciaa1248
Same direction. This prospective serial sampling of 70 patients revealed clinically relevant cycle thresholds (Ct, “viral load”), namely a Ct of 24 (“high viral load”), 34, and > 40 (“negative”) that occurred 9, 26, and 36 days after symptom onset. Of note, race, gender, or corticosteroids did not appear to influence RNA-positivity. A retrospective analysis of 180 patients revealed that initial Ct did not correlate with requirement for admission or intensive care.
22 August
Link-Gelles R, DellaGrotta AL, Molina C, et al. Limited Secondary Transmission of SARS-CoV-2 in Child Care Programs — Rhode Island, June 1–July 31, 2020. MMWR Morb Mortal Wkly Rep. ePub: 21 August 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6934e2
Ruth Link-Gelles et al. report a possible secondary transmission in four of the 666 child-care programs in Rhode Island that were allowed to reopen. The apparent absence of secondary transmission within the other 662 child-care programs was likely the result of efforts to contain SARS-CoV-2 transmission, in particular maximum class sizes and use of face masks for adults. The authors conclude that adherence to current CDC recommendations remains critical to reducing transmission in child-care settings, including wearing of masks by adults, limiting mixing between established student-teacher groups, staying home when ill, and cleaning and disinfecting frequently touched surfaces.
21 August
Hoehl S, Karaca O, Kohmer M, et al. Assessment of SARS-CoV-2 Transmission on an International Flight and Among a Tourist Group. JAMA Netw Open August 18, 2020, 3(8). Full-text: https://doi.org/10.1001/jamanetworkopen.2020.18044
Two likely SARS-CoV-2 transmissions on a 4.5-hour flight from Tel Aviv to Frankfurt, with 7 index cases. Both passengers were seated within two rows of an index case. According to the authors, it could be speculated that the rate may have been reduced further had the passengers worn masks.
Bhaskar ME, Arun S. SARS-CoV-2 Infection Among Community Health Workers in India Before and After Use of Face Shields. JAMA August 17, 2020. Full-text: https://doi.org/10.1001/jama.2020.15586
This observational study describes transmission before and after the use of face shields (made of polyethylene terephthalate) in health workers in Chennai, India. Before the introduction of face shields, 12/62 workers were infected, while visiting 5,880 homes with 31,164 persons (222 positive for SARS-CoV-2). After the introduction, among 50 workers (previously uninfected) who continued to provide counseling, visiting 18,228 homes with 118,428 persons (2682 positive), no infection occurred.
20 August
Chambers C, Krogstad P, Betrand K, et al. Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women. JAMA August 19, 2020. Full-text: https://doi.org/10.1001/jama.2020.15580
There are some case reports on the detection of SARS-CoV-2 in breast milk. Christina Chambers and colleagues examined 64 breast milk samples from 18 infected women. Although SARS-CoV-2 RNA was detected in one milk sample, the viral culture for that sample was negative. These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant.
19 August
Asadi S, Gaaloul ben Hnia N, Barre RS, et al. Influenza A virus is transmissible via aerosolized fomites. Nat Commun 11, 4062 (2020). Full-text: https://doi.org/10.1038/s41467-020-17888-w
SARS-CoV-2 can be transmitted via droplets, fomites and possibly aerosol. Will we need to get accustomed to a fourth transmission route, aerosolized fomites? That’s what Nicole Bouvier and colleagues suggest, although for now only for influenza A virus. They show that dried influenza virus remains viable in the environment, on materials like paper tissues and on the bodies of living animals, long enough to be aerosolized on non-respiratory dust particles that can transmit infection through the air to new mammalian hosts. Will we soon see a paper about SARS-CoV-2 transmission via aerosolized fomites?
18 August
Bui DP, McCaffrey K, Friedrichs M, et al. Racial and Ethnic Disparities Among COVID-19 Cases in Workplace Outbreaks by Industry Sector — Utah, March 6–June 5, 2020. MMWR Morb Mortal Wkly Rep. ePub: 17 August 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6933e3
We need to understand which types of work have the highest exposure risk for SARS-CoV-2. In Utah, US, from March to June 2020, approximately 12% of confirmed COVID-19 cases were associated with workplace outbreaks. The 210 workplace outbreaks occurred in 15 of 20 industry sectors; nearly half of all workplace outbreaks occurred in three sectors: Manufacturing (43; 20%), Construction (32; 15%) and the Wholesale Trade (29; 14%); 58% (806 of 1,389) of workplace outbreak-associated cases occurred in these three sectors. David Bui and colleagues recommend that mitigation strategies should be culturally and linguistically responsive to racial/ethnic minority workers disproportionately affected by COVID-19.
17 August
Lewis NM, Chu VT, Ye D, et al. Household Transmission of SARS-CoV-2 in the United States. Clinical Infectious Diseases, 16 August 2020. Full-text: https://doi.org/10.1093/cid/ciaa1166
Nathaniel M Lewis and colleagues sought to estimate the household secondary infection rate (SIR) of SARS-CoV-2 and evaluate potential risk factors for secondary infection among 58 households in Utah and Wisconsin. Fifty-two of 188 household contacts acquired secondary infections (SIR: 28%, 95% CI: 22–34%). Of note, household contacts to COVID-19 patients with immunocompromised conditions had increased odds of infection (OR: 15.9, 95% CI: 2.4–106.9) as well as household contacts who themselves had diabetes mellitus (OR: 7.1, 95% CI: 1.2–42.5).
Garigliany M, Van Laere A-S, Clercx C, Giet D, Escriou N, Huon C, et al. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg Infect Dis. 2020 Dec [date cited]. Full-text: https://doi.org/10.3201/eid2612.202223
Mutien Garigliany from Liège, Belgium, and colleagues report a human-to-cat transmission. A household cat was productively infected with the SARS-CoV-2 virus excreted by its owner, and the infection caused a non-fatal but nevertheless severe disease.
14 August
Luo L, Liu D, Liao X, et al. Contact Settings and Risk for Transmission in 3410 Close Contacts of Patients With COVID-19 in Guangzhou, China: A Prospective Cohort Study. Ann Intern Med. 2020 Aug 13. PubMed: https://pubmed.gov/32790510. Full-text: https://doi.org/10.7326/M20-2671
Chen Mao and colleagues traced 3410 close contacts of 391 SARS-CoV-2 infected index cases between 13 January and 6 March 2020. 127 contacts (3.7%) were secondarily infected. Compared with the household setting (10.3%), the secondary attack rate was lower for exposures in healthcare settings (1.0%) and on public transportation (0.1%). Interestingly, although not unexpectedly, the secondary attack rate increased with the severity of index cases, from 0.3% for asymptomatic to 3.3% for mild, 5.6% for moderate, and 6.2% for severe or critical cases. Index cases with expectoration were associated with higher risk for secondary infection (13.6% vs. 3.0% for index cases without expectoration).
13 August
Sickbert-Bennett EE, Samet J, Clapp PW, et al. Filtration Efficiency of Hospital Face Mask Alternatives Available for Use During the COVID-19 Pandemic. JAMA Intern Med. Published online August 11, 2020. Full-text: https://doi.org/10.1001/jamainternmed.2020.4221
Face masks can be old, but they must fit. This quality improvement study evaluating 29 face mask alternatives found that expired N95 respirators and sterilized, used N95 respirators can be used when new N95 respirators are not available. Other alternatives may provide less effective filtration. The performance of N95 respirators in the wrong size had a slightly decreased performance.
Huong NQ, Nga NTT, Long NV, et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013-2014. PLoS One. 2020 Aug 10;15(8):e0237129. PubMed: https://pubmed.gov/32776964. Full-text: https://doi.org/10.1371/journal.pone.0237129
These researchers from Vietnam identified six known coronaviruses in bats and rodents, clustered in three Coronaviridae genera. Most notably among field rats, the odds of coronavirus RNA detection were highest in field rats sold and served in restaurants (55.6%, 84/151). The mixing of multiple coronaviruses, and their apparent amplification along the wildlife supply chain into restaurants, suggests maximal risk for end consumers and likely underpins the mechanisms of zoonotic spillover to people.
Greenhalgh T, Knight M, A’Court, et al. Management of post-acute covid-19 in primary care. BMJ 2020; 370. Full-text: https://doi.org/10.1136/bmj.m3026
Nice and pragmatic summary of the management of COVID-19 after the first three weeks (which is currently based on limited evidence). Key messages: Approximately 10% of people experience prolonged illness. Many such patients recover spontaneously (if slowly) with holistic support, rest, symptomatic treatment, and gradual increase in activity. Home pulse oximetry can be helpful in monitoring breathlessness.
12 August
Lednicky JA, Lauzardo M, Fan ZH, et al. Viable SARS-CoV-2 in the air of a hospital room 1 with COVID-19 patients. medRxiv 2020, posted 4 August. Pre-print: https://www.medrxiv.org/content/10.1101/2020.08.03.20167395v1
John A. Lednicky and colleagues isolated viable virus from air samples collected 2 to 4.8 meters away from two COVID-19 patients. The genome sequence of the SARS-CoV-2 strain isolated was identical to that isolated from the NP swab from the patient with an active infection. Estimates of viable viral concentrations ranged from 6 to 74 TCID50 units/L of air. This paper has not yet been peer-reviewed.
Chagla Z, Hota S, Khan S, Mertz D, and the International Hospital and Community Epidemiology Group. Airborne Transmission of COVID-19. Clin Infect Dis 2020, published 11 August. Full-text: https://doi.org/10.1093/cid/ciaa1118
Zain Chagla and colleagues discuss the paper by Morawska L, Milton DK, It is Time to Address Airborne Transmission of COVID-19 (Clin Infect Dis 2020, 6 July). They agree that there is potential for the transmission by aerosols, especially in poorly ventilated indoor crowded environments. However, they argue that the main mode of transmission of SARS-CoV-2 is short range through droplets and close contact. Explore this one-page comment to see how the debate continues.
Bigelow BF, Tang O, Toci GR, et al. Transmission of SARS-CoV-2 Involving Residents Receiving Dialysis in a Nursing Home — Maryland, April 2020. MMWR Morb Mortal Wkly Rep. ePub: 11 August 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6932e4
Nursing home residents who receive hemodialysis are at higher risk for SARS-CoV-2 infections. Benjamin Bigelow and colleagues investigated a COVID-19 outbreak in a Maryland nursing home. The prevalence of infection among residents undergoing dialysis was 47% (15 of 32) as compared to those not receiving dialysis (16%; 22 of 138) (p < 0.001). The authors recommend strict control practices throughout the dialysis process, e.g., transportation, time spent in waiting areas, spacing of machines, and cohorting.
9 August
Totura A, Livingston V, Frick O, Dyer D, Nichols D, Nalca A. Small particle aerosol exposure of African green monkeys to MERS-CoV as a model for highly pathogenic coronavirus infection. Emerg Infect Dis 2020. Published August 2020. Full-text: https://doi.org/10.3201/eid2612.201664
For the initial development of a MERS-CoV primate model, Allison Totura and colleagues exposed 12 African green monkeys to 103, 104, or 105 PFU target doses of aerosolized MERS-CoV. Clinical disease signs that replicated human cases of MERS were observed in all groups but were most pronounced in the group that received the highest dose of MERS-CoV. It would be interesting to investigate if a dose-dependent increase of respiratory disease signs can be replicated in a SARS-CoV-2 animal model.
7 August
Klompas M, Baker MA, Rhee C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA. 2020 Aug 4;324(5):441-442. PubMed: https://pubmed.gov/32749495 . Full-text: https://doi.org/10.1001/jama.2020.12458
Brief review. It is impossible to conclude that aerosol-based transmission never occurs, write Michael Klompas and colleagues, but the balance of currently available evidence suggests that long-range aerosol-based transmission is not the dominant mode of SARS-CoV-2 transmission.
Joonaki E, Hassanpouryouzband A, Heldt Cl, et al. Surface Chemistry Can Unlock Drivers of Surface Stability of SARS-CoV-2 in Variety of Environmental Conditions. Chem, August 06, 2020. Full-text: https://doi.org/10.1016/j.chempr.2020.08.001
Nice overview of existing knowledge concerning viral spread, molecular structure of SARS-CoV-2, and the stability of the virus surface. Edris Joonaki and colleagues discuss potential drivers of the SARS-CoV-2 surface adsorption and stability in various environmental conditions.
6 August
Hu M, Lin H, Wang J, et al. The risk of COVID-19 transmission in train passengers: an epidemiological and modelling study. Clin Infect Dis 2020, published 29 July. Full-text: https://doi.org/10.1093/cid/ciaa1057
How risky is train traveling in the COVID-19 era? To answer this question, analyze passengers in Chinese high-speed trains. Jinfeng Wang and colleagues quantified the transmission risk using data from 2,334 index patients and 72,093 close contacts who had co-travel times of 0–8 hours from 19 December 2019 through 6 March 2020. Unsurprisingly, travelers adjacent to an index patient had the highest attack rate (3.5%) and the attack rate decreased with increasing distance, but increased with increasing co-travel time. The overall attack rate of passengers with close contact with index patients was 0.32%. The author’s conclusion: during COVID outbreaks, when travelling on public transportation in confined spaces such as trains, increase seat distance and reduce passenger density.
5 August
Macartney K, Quinn HE, Pillbury AJ. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health, August 03, 2020. Full-text: https://doi.org/10.1016/S2352-4642(20)30251-0
Transmission in schools may be less frequent than expected. This group has analyzed 15 schools and ten ECEC (early childhood education and care) settings which had children (n=12) or adults (n=15) attending while infectious, with 1,448 contacts monitored. Of these, 633 (44%) had nucleic acid testing or antibody testing, with 18 secondary cases identified (attack rate 1.2%). Five secondary cases (three children; two adults) were identified (attack rate 0.5%; 5/914) in three schools. No secondary transmission occurred in nine of ten ECEC settings among 497 contacts. However, one outbreak in an ECEC setting involved transmission to six adults and seven children (attack rate 35%; 13/37).
Valentine R, Valentine D, Valentine JL. Relationship of George Floyd protests to increases in COVID-19 cases using event study methodology. Journal of Public Health, August 5, 2020. Full-text: https://doi.org/10.1093/pubmed/fdaa127
Best author list of the day. Randall, Dawn and Jimmie L. Valentine (from different institutions) show that in 6/8 US cities in which protestors in the tens of thousands were reported, infection rate growth was positive and significant. The Valentines argue that to slow the spread of COVID-19, CDC guidelines must be followed in protest situations. Well, it’s not that easy when you are angry.
3 August
Szablewski CM, Chang KT, Brown MM, et al. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp — Georgia, June 2020. MMWR Morb Mortal Wkly Rep. ePub: 31 July 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6931e1
Mid-June 2020. An overnight camp in Georgia (camp A) with trainees, staff members and campers. Wearing cloth masks for campers and opening windows and doors for increased ventilation in buildings were not required. (Cloth masks were required only for staff members.) Camp attendees engaged in a variety of indoor and outdoor activities, including daily vigorous singing and cheering. Of a total of 597 Georgia residents attendending camp A, test results were available for 344 (58%) attendees; among these 260 (76%) were positive. The overall attack rate was 44% (260 of 597), 51% among those aged 6–10 years, 44% among those aged 11–17 years, and 33% among those aged 18–21 years. Attack rates increased with increasing length of time spent at the camp, with staff members having the highest attack rate (56%).
30 July
Chen Y, Qin G, Chen J, et al. Comparison of Face-Touching Behaviors Before and During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open 2020;3(7):e2016924. https://doi.org/10.1001/jamanetworkopen.2020.16924
Is wearing face masks really associated with reduced face-touching behaviors? To answer the question, Xing Li and colleagues from Sun Yat-sen University, Guangzhou, China, used videos recorded in public transportation stations, streets, and parks among the general population in China, Japan, South Korea, Western Europe (ie, England, France, Germany, Spain, and Italy), and the US to analyze mask-wearing and face-touching behavior in public areas. The authors found that mask wearing was associated with reduced face-touching behavior, especially touching of the eyes, nose, and mouth. They conclude that the reduction of face-touching behaviors by mask wearing could contribute to curbing the COVID-19 pandemic. Excellent news for the coming months.
Santarpia JL, Rivera DN, Herrera VL et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep 10, 12732 (2020). Full-text: https://doi.org/10.1038/s41598-020-69286-3
After evacuation from the Diamond Princess cruise ship in March 2020, 11 were admitted to a hospital in Nebraska, two in a biocontainment unit and 9 in a quarantine unit. Key features of both units included: (1) individual rooms with private bathrooms; (2) negative-pressure rooms (> 12 ACH) and negative-pressure hallways; (3) key-card access control; (4) unit-specific infection prevention and control (IPC) protocols including hand hygiene and changing of gloves between rooms; and (5) personal protective equipment (PPE) for staff that included contact and aerosol protection. Joshua Santarpia and colleagues collected air and surface samples to examine viral shedding from isolated individuals and detected viral contamination among all samples. Their data suggest that SARS-CoV-2 environmental contamination around COVID-19 patients is extensive, and hospital IPC procedures should account for the risk of fomite, and potentially airborne, transmission of the virus.
29 July
Sekizuka T, Itokawa K, Kageyama T, et al. Haplotype networks of SARS-CoV-2 infections in the Diamond Princess cruise ship outbreak. PNAS 2020, published 28 July. Full-text: https://doi.org/10.1073/pnas.2006824117
We remember the Diamond Princess: a cruise ship put under quarantine off of Yokohama, Japan, in early February. Of around 3,700 people on board, more than 700 people became infected with SARS-CoV-2 and seven patients died. Now, after whole-genome sequencing of SARS-CoV-2 and a network/phylogeny analysis of the outbreak, Makoto Kuroda and colleagues conclude that there was a single introduction of SARS-CoV-2, which disseminated among passengers on the ship through possible mass-gathering events in the recreational areas where people dance, sing, watch performances, or shop.
28 July
Riediker M, Tsai D. Estimation of Viral Aerosol Emissions From Simulated Individuals With Asymptomatic to Moderate Coronavirus Disease 2019. JAMA Netw Open 2020;3(7):e2013807. Full-text: https://doi.org/10.1001/jamanetworkopen.2020.13807
In this modeling study, Michael Riediker from the Swiss Centre for Occupational and Environmental Health in Winterthur and Dai-Hua Tsai from the University Hospital of Psychiatry in Zurich, Switzerland, it is estimated that viral load concentrations in a room with an individual who was coughing frequently were very high, with a maximum of 7.44 million copies/m3 from an individual who was a high emitter. However, regular breathing from an individual who was a high emitter was modeled to result in lower room concentrations of up to 1248 copies/m3. They conclude that the estimated infectious risk posed by a person with typical viral load who breathes normally was low and that only a few people with very high viral load posed an infection risk in the poorly ventilated closed environment simulated in this study.
27 July
Patterson EI, Elia G, Grassi A, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. bioRxiv 23 July 2020. Full-text: https://doi.org/10.1101/2020.07.21.214346
Nicola Decaro and colleagues assess SARS-CoV-2 infection in 817 companion animals in northern Italy at the height of the spring 2020 epidemic. Although no animals tested PCR positive, 3.4% of dogs and 3.9% of cats had measurable SARS-CoV-2 neutralizing antibody titers, with dogs from COVID-19 positive households being significantly more likely to test positive than those from COVID-19 negative households. From their experience, the authors conclude that it is unlikely that infected pets play an active role in SARS-CoV-2 transmission to humans. Only under special circumstances, such as the high animal population densities encountered on infected mink farms, animal-to-human transmission might be likely.
25 July
Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med, July 24, 2020. Full-text: https://doi.org/10.1016/S2213-2600(20)30323-4
Is there really evidence that some pathogens are carried only in large droplets? Or would cough aerosols and exhaled breath from patients with various respiratory infections show striking similarities in aerosol size distributions? In case of doubt, how would you protect your family and yourself?
Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020 separator commenting unavailable. Eurosurveill 2020, Volume 25, Issue 29, 23 July. Full-text: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.29.2001352
After two months of lockdown, schools in Israel re-opened on 17 May. Ten days later, two cases of SARS-CoV-2 infection were diagnosed in a high school in Jerusalem; the two cases were epidemiologically not linked. Testing of the school community revealed 153 students (attack rate: 13.2%) and 25 staff members (16.6%) who were SARS-CoV-2 positive. Overall, some 260 persons were infected (students, staff members, relatives and friends).
As September approaches, health authorities in other countries should take note. In the present study, the mass COVID-19 transmission occurred when teenage students returned to their regular classes after a 2-month closure. An extreme heatwave (on 19 May) with temperatures rising to 40 °C and above led to an exemption from facemasks for three days (19–21 May) and continuous air-conditioning. Classes in the first affected school had more than 30 students.
The authors remind us that COVID-19 prevention in schools involves
- Studying in small groups
- Minimizing student mixing in activities and transportation
- The wearing of facemasks by teachers and parents
- Keeping physical distance and practicing hand hygiene
- Avoiding school attendance at any sign of illness
- Learning from home if possible to reduce the need for class attendance
- Organize outdoors classes in selected cases
Remember the ‘three Cs’: closed spaces with poor ventilation, crowded places and close-contact settings.
24 July
Günther T, Czech-Sioli M, Indenbirken D, et al. Investigation of a superspreading event preceding the largest meat processing. Pre-print available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3654517
In June, more than 1,400 employees at a meat-processing plant (MPP) in Germany were infected with SARS-CoV-2. Now a research group led by virologist Melanie Brinkmann (Helmholtz Center for Infection Research, Braunschweig) reconstructed how the virus was transmitted in the company. The first employees who became infected worked the early shift (147 workers), mostly in a fixed position on the conveyor belt. The evaluation of these positions showed that the risk of infection was greatest within a distance of eight meters from the first infected individual. In order words: a distance of 1.5 or two meters, which is currently thought (and instituted!) as relatively safe in most situations, was far from sufficient. The authors conclude that climate conditions (10° C ambient air temperature) and airflow are important factors that can promote spread of SARS-CoV-2 via distances of more than 8 meters. These findings may have far-reaching implications for pandemic mitigation strategies in industrial workplace settings.
Yamagishi T, Ohnishi M, Matsunaga N, et al. Environmental sampling for severe acute respiratory syndrome coronavirus 2 during COVID-19 outbreak in the Diamond Princess cruise ship. J Infect Dis. 2020 Jul 21:jiaa437. PubMed: https://pubmed.gov/32691828. Full-text: https://doi.org/10.1093/infdis/jiaa437
In the early epidemic in Japan, many infections occurred among the passengers and crew members on board the Diamond Princess cruise ship in February, 2020. By March 1, 2020, there were approximately 700 individuals with laboratory-detected SARS-CoV-2 infection (see the previous articles by Russell et al., Yamagishi et al. and Tabata et al.). The authors performed environmental sampling on the Diamond Princess cruise ship on 22-23 February 2020 (prior to disinfection of the vessel and while some passengers and crew members remained aboard) and obtained specimens from cabins in which confirmed COVID-19 cases stayed (case cabins), cabins with no confirmed case at any point (non-case cabins), and common areas. SARS-CoV-2 RNA was detected from 58 out of 601 samples (10%) from case cabins 1-17 days after the cabins were vacated, but not from non-case cabins. There was no difference in the detection proportion between cabins for symptomatic (15%, 28/189) and asymptomatic cases (21%, 28/131). No SARS-CoV-2 virus was isolated from any of the samples. The authors conclude that transmission risk of SARS-CoV-2 from symptomatic and asymptomatic patients may be similar and environmental surfaces could be involved in viral transmission.
21 July
Stewart CL, Thornblade LW, Diamond DJ, Fong Y, Melstrom LG. Personal Protective Equipment and COVID-19: A Review for Surgeons. Ann Surg. 2020 Aug;272(2):e132-e138. PubMed: https://pubmed.gov/32675516. Full-text: https://doi.org/10.1097/SLA.0000000000003991
Are you a surgeon? Then your particular medical association has been using personal protective equipment (PPE) for more than a century. This review addresses both the mechanism of SARS-CoV-2 transmission and the capabilities of PPE in the perioperative COVID-19 setting.
Plautz J. Is it safe to strike up the band in a time of coronavirus? Science, 17 July 2020. Full-text: https://www.sciencemag.org/news/2020/07/it-safe-strike-band-time-coronavirus
Is keeping 2 meters away enough to stay safe from a trumpet at full blast? Try it, find out! Introduce five student musicians – a soprano singer and clarinet, flute, French horn, and trumpet players — in a clean room one at a time and let them perform a short solo piece.
20 July
Park YJ, Choe YJ, Park O, et al. Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg Infect Dis October 2020. Full-text: https://wwwnc.cdc.gov/eid/article/26/10/20-1315_article
The authors analyzed 59,073 contacts of 5,706 COVID-19 index patients. Of 10,592 household contacts, 11.8% had COVID-19; rates were higher for contacts of children than adults. Of 48,481 non-household contacts, 1.9% had COVID-19. Interestingly, the highest COVID-19 rate (18.6%) was found for household contacts of school-aged children and the lowest (5.3%) for household contacts of children 0–9 years in the middle of school closure.
17 July
Hendrix MJ, Walde C, Findley K, Trotman R. Absence of Apparent Transmission of SARS-CoV-2 from Two Stylists After Exposure at a Hair Salon with a Universal Face Covering Policy — Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep. 14 July 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6928e2
Have we ever mentioned masks? Among 139 clients exposed to two symptomatic hair stylists with confirmed COVID-19 while both the stylists and the clients wore face masks, not a single symptomatic secondary case was observed; among 67 clients tested for SARS-CoV-2, all tests were negative. At least one hair stylist was infectious: all four close household contacts (presumably without masks) became ill.
Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association Between Universal Masking in a Health Care System and SARS-CoV-2 Positivity Among Health Care Workers. JAMA. 2020 Jul 14. PubMed: https://pubmed.gov/32663246. Full-text: https://doi.org/10.1001/jama.2020.12897
Again, universal masking: in March 2020, the Mass General Brigham, the largest health care system in Massachusetts (12 hospitals, > 75,000 employees), implemented universal masking of all HCWs and patients with surgical masks. During the preintervention period, the SARS-CoV-2 positivity rate increased exponentially, with a case doubling time of 3.6 days. During the intervention period, the positivity rate decreased linearly from 14.65% to 11.46%, with a weighted mean decline of 0.49% per day and a net slope change of 1.65% additional decline per day compared with the preintervention period.
Contejean A, Leporrier J, Canouï E, et al. Comparing dynamics and determinants of SARS-CoV-2 transmissions among health care workers of adult and pediatric settings in central Paris. Clin Infect Dis. 2020 Jul 15:ciaa977. PubMed: https://pubmed.gov/32663849. Full-text: https://doi.org/10.1093/cid/ciaa977
This prospective study compared a 1,500-bed adult and a 600-bed pediatric setting of a university hospital located in central Paris. From February 24th until April 10th, 2020, all symptomatic HCW were screened. Attack rates were of 3.2% and 2.3% in the adult and pediatric setting, respectively (p = 0.0022). In the adult setting, HCW more frequently reported exposure to COVID-19 patients without PPE (25% versus 15%, p = 0.046). The total number of HCW cases peaked on March 23rd, then decreased slowly, concomitantly with a continuous increase in preventive measures (including universal medical masking and PPE). Residual transmissions were related to exposures with undiagnosed patients or colleagues but not to contacts with children attending out-of-home care facilities.
Brooks JT, Butler JC, Redfield RR. Universal Masking to Prevent SARS-CoV-2 Transmission—The Time Is Now. JAMA July 14, 2020. Full-text: https://doi.org/10.1001/jama.2020.13107
See title. Data is clear now. First, public health officials need to ensure that the public understands clearly when and how to wear cloth face coverings properly. Second, innovation is needed to extend physical comfort and ease of use. Third, the public needs consistent, clear, and appealing messaging that normalizes community masking. According to the authors, broad adoption of cloth face coverings is a civic duty, a small adaption in our daily lives reliant on a highly effective low-tech solution that can help turn the tide.
16 July
Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun2020. Full-text: https://doi.org/10.1038/s41467-020-17436-6
Maybe the first documented case of transplacental transmission. French doctors report on a 23-year-old COVID-19 patient who gave birth by cesarean section to a baby found to have the infection. The viral load was much higher in the placental tissue than in the amniotic fluid or maternal blood: this suggests the presence of the virus in placental cells, which is consistent with findings of inflammation seen at histological examination. Good news: baby is fine.
13 July
Xie W, Campbell S, Zhang W. Working memory capacity predicts individual differences in social-distancing compliance during the COVID-19 pandemic in the United States. Proc Natl Acad Sci U S A. 2020 Jul 10:202008868. PubMed: https://pubmed.gov/32651280. Full-text: https://doi.org/10.1073/pnas.2008868117
Among 850 US residents participating in a survey, the authors found that social distancing compliance could be predicted by individual differences in working memory (WM) capacity. WM retains a limited amount of information over a short period of time at the service of other ongoing mental activities. Its limited capacity constrains our mental functions, such that higher WM capacity is often associated with better cognitive and affective outcomes. Of note, the unique contribution of WM capacity to the individual differences in social distancing compliance could not be explained by other psychological and socioeconomic factors (e.g., moods, personality, education, and income levels). The message that the authors hide using scientific language can be said more clearly: if you see a guy sitting in the bus not wearing a mask: poor idiot, don’t get closer. His WM capacity is poor.
11 July
Dorfman D, Raz M. Mask Exemptions During the COVID-19 Pandemic—A New Frontier for Clinicians. JAMA Health Forum July 10, 2020. Full-text: https://jamanetwork.com/channels/health-forum/fullarticle/2768376
While masking remains contentious, there is bipartisan agreement among policy makers that medical exemptions for masking are necessary and appropriate. Yet there is a dearth of guidance for clinicians on how to approach a request for an exemption. The authors analyze the medical and legal standards to guide this debate. In this evidence-free zone, clinicians must make individual determinations as to whether a patient should be exempt from mask wearing. There is no obligation to provide a mask exemption to patients if it is not medically warranted.
10 July
Rockett RJ, Arnott A, Lam C, et al. Revealing COVID-19 transmission in Australia by SARS-CoV-2 genome sequencing and agent-based modeling. Nat Med 2020 Jul 9. PubMed: https://pubmed.gov/32647358. Full-text: https://doi.org/10.1038/s41591-020-1000-7
These researchers examined the added value of near real-time genome sequencing of SARS-CoV-2 in a subpopulation of infected patients during the first 10 weeks of COVID-19 containment in Australia. Genomic evidence was used to cluster 38.7% (81 out of 209) of cases for which the available epidemiological data could not identify direct links. This included clustering 12.4% (26 out of 209) of cases with a history of recent arrival from overseas with other cases without a travel history and 5.3% (11/209) of locally acquired cases with unknown epidemiological links. Twenty-two (10.5%) of the 209 cases were epidemiologically classified as ‘locally acquired—contact not identified’.
Mueller AV, Eden MJ, Oakes JM, et al. Quantitative Method for Comparative Assessment of Particle Removal Efficiency of Fabric Masks as Alternatives to Standard Surgical Masks for PPE. Matter July 09, 2020. Full-text: https://doi.org/10.1016/j.matt.2020.07.006
The effectiveness of masks to protect wearers from airborne particles is known to be a function of both materials and fit. The authors present a rapid testing protocol for evaluation of loose-fitting type masks to provide quantitative, intercomparable data for particle removal efficacy of masks made with different types of fabrics and with different designs/fits, independently providing an assessment of the quality of the mask fit and the material used. Commercial surgical masks marketed for medical use had mean particle removal efficiencies from 50-75% when worn as designed but up to 90% when close fitting to the face under a nylon layer. Cloth masks tested had widely varying mean particle removal efficiencies (< 30% to near 90%), with some cloth masks achieving similar particle removal efficiencies as commercial surgical masks.
9 July
Chen J, He H, Cheng W, et al. Potential transmission of SARS-CoV-2 on a flight from Singapore to Hanghzou, China: An epidemiological investigation. J Trav Med 2020, Jul 6, 2020. Full-text: https://doi.org/10.1016/j.tmaid.2020.101816
Among 335 passengers on a flight from Singapore to Hangzhou in China (a Boeing 787, 5-hour flight, seat occupancy 89%), a total of 16 COVID-19 patients were diagnosed among all passengers, yielding an attack rate of 4.8%. However, after careful investigation, only one case was identified who appears to have become infected during the flight. He was seated near four infected passengers from Wuhan for approximately an hour (he had moved a seat) and did not wear his facemask correctly during the flight. The sources of infection in the other 15 passengers were complex and the passengers could have acquired their infections in Wuhan before the tour, or during the group tour before boarding.
8 July
Zhou J, Otter JA, Price JR, et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis. 2020 Jul 8:ciaa905. PubMed: https://pubmed.gov/32634826. Full-text: https://doi.org/10.1093/cid/ciaa905
Cross-sectional observational study in a multi-site London hospital. Air and surface samples were collected from several areas. Viral RNA was detected on 114/218 (52.3%) of surfaces and 14/31 (38.7%) air samples but no virus was cultured. Viral RNA was more likely to be found in areas immediately occupied by COVID-19 patients than in other areas. The high PCR Ct value for all samples (> 30) indicated that the virus would not be culturable.
Schlottau K, Rissmann M, Graaf A, et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe July 07, 2020. Full-text: https://doi.org/10.1016/S2666-5247(20)30089-6
Lucky pigs. The authors intranasally inoculated twelve fruit bats (Rousettus aegyptiacus), 12 ferrets (Mustela putorius), pigs (Sus scrofa domesticus), and 20 chickens (Gallus gallus domesticus) with TCID50 of a SARS-CoV-2 isolate per animal. Pigs and chickens could not be infected intranasally or oculo-oronasally by SARS-CoV-2, whereas fruit bats showed characteristics of a reservoir host. Virus replication in ferrets resembled a subclinical human infection with efficient spread.
7 July
Morawska L, Milton DK. It is Time to Address Airborne Transmission of COVID-19. Clinical Infectious Diseases, July 6, 2020. Full-text: https://doi.org/10.1093/cid/ciaa939 l (Important)
In their comment, the authors appeal to the medical community and to all relevant national and international bodies to recognize the potential for airborne spread of COVID-19. Given the significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale), the authors are advocating for the use of preventive measures. This includes sufficient and effective ventilation (supply clean outdoor air, minimize recirculating air) particularly in public buildings, workplace environments, schools, hospitals, and aged care homes, but also supplement general ventilation with airborne infection control (such as local exhaust, high efficiency air filtration, and germicidal ultraviolet lights). Third, overcrowding has to be avoided, particularly in public transport and public buildings
Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among Frontline Healthcare Personnel During the First Month of Caring for COVID-19 Patients – Nashville, Tennessee. Clin Infect Dis. 2020 Jul 6. PubMed: https://pubmed.gov/32628750. Full-text: https://doi.org/10.1093/cid/ciaa936
Among 249 HCW who worked in hospital units with COVID-19 patients for one month, 19 (7.6%) tested positive for SARS-CoV-2 antibodies. Only 11/19 (57.9%) reported symptoms of a prior illness, suggesting asymptomatic HCW could be an important source of SARS-CoV-2 transmission.
5 July
Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Inf Dis July 03, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30561-2
A note of caution, to curb excesses that become counterproductive. According to the author, the chance of transmission through inanimate surfaces is very small, and only in instances where an infected person coughs or sneezes on the surface, and someone else touches that surface soon after the cough or sneeze (within 1–2 h). Although periodically disinfecting surfaces and use of gloves are reasonable precautions especially in hospitals, he believes that fomites that have not been in contact with an infected carrier for many hours do not pose a measurable risk of transmission.
4 July
Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg Dis. 2020 Jun 30. PubMed: https://pubmed.gov/32603505. Full-text: https://doi.org/10.1111/tbed.13707
A comprehensive review of the available data (by May 21, 2020) on the stability of coronaviruses, including SARS-CoV-2, from previous reports, to help understand its environmental survival.
Edwards SJL, Santini JM. Anthroponotic risk of SARS-CoV-2, precautionary mitigation, and outbreak management. Lancet Microbe, July 02, 2020. Full-text: https://doi.org/10.1016/S2666-5247(20)30086-0
Important comment on the evidence of infection of animals with SARS-CoV-2 that has been shown experimentally both in vivo and in vitro for mammals including monkeys, cats, ferrets, rabbits, foxes, and hamsters, while bioinformatic studies also predict infectivity of pigs and wild boar among other mammals. According to the authors, we should also consider the potential for transmissibility, not just infection.
Sikkema RS, Niewenhuijse DF, O’Toole A, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Inf Dis, July 02, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30527-2
Social events outside the hospital. In this cross-sectional study at three hospitals located in the south of the Netherlands, from 50 HCWs (and ten patients), complete and near-complete genome sequences were analyzed. Most sequences were grouped into three clusters, with two clusters showing local circulation within the region. The genomic diversity recorded was consistent with multiple introductions through community-acquired infections, and some local amplification related to specific social events in the community, rather than widespread within-hospital transmission. Thus, data do not support widespread nosocomial transmission as the source of infection in patients or health-care workers.
3 July
Guo L, Zhao S, Li W, et al. Absence of SARS-CoV-2 in Semen of a COVID-19 Patient Cohort. Andrology. 2020 Jun 29. PubMed: https://pubmed.gov/32598557. Full-text: https://doi.org/10.1111/andr.12848
No virus in the semen: all of 23 brave patients with SARS-CoV-2 infections (12 of them still positive in sputum and fecal specimens) tested negative for SARS‐CoV‐2 RNA in semen specimens.
Bastug A, Hanifehnezhad A, Tayman C, et al. Virolactia in an Asymptomatic Mother with COVID-19. Breastfeed Med. 2020 Jul 1. PubMed: https://pubmed.gov/32614251. Full-text: https://doi.org/10.1089/bfm.2020.0161
Another case report of a pregnant woman with subclinical COVID-19 whose breast milk sample obtained after delivery tested positive for SARS-CoV-2 by RT-PCR. In addition, although an initial nasopharyngeal swab (NPS) sample from the neonate resulted negative, neonatal NPS, stool, and blood samples obtained after breastfeeding were all positive in real-time RT-PCR assay.
1 July
Abbas M, Pittet D. Surfing the COVID-19 scientific wave. Lancet June 30, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30558-2
Harsh, relentless (maybe justified?) critical letter on the methodological flaws of the experiment of visualizing speech-generated oral fluid droplets (see below). The authors are “surprised that experiments in one person were published in a leading scientific journal” and state that the experiment had “more to do with sialoquence (spraying saliva when speaking) than with SARS-CoV-2”.
Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020; 382: 2061-2063. Full-text: https://www.nejm.org/doi/full/10.1056/nejmc2007800
30 June
Dau NQ, Lau H, Skinner C. Why N95 Should Be the Standard for All COVID-19 Inpatient Care. Ann Int Med 2020, Jun 29. Full-text: https://doi.org/10.7326/M20-2623
Important viewpoint emphasizing that N95 respirators achieve better filtration of airborne particles than medical masks if used properly and continuously. According to the authors, guideline recommendations that do not support N95 use for all inpatient COVID-19 management should consider reevaluating existing data or at least acknowledge the issues raised.
Oosterhoff B, Palmer CA. Attitudes and Psychological Factors Associated With News Monitoring, Social Distancing, Disinfecting, and Hoarding Behaviors Among US Adolescents During the Coronavirus Disease 2019 Pandemic. JAMA Pediatr. Published online June 29, 2020. Full-text: https://doi.org/10.1001/jamapediatrics.2020.1876
Interesting survey on 770 adolescents’ beliefs about COVID-19 and community attachment as well as attitudes and psychological factors that inform their response to the pandemic. Many teens reported not engaging in pure social distancing (69%), but they were monitoring the news (89%) and disinfecting daily (88%). Some teens reported hoarding (20%). Greater social responsibility was associated with more disinfecting and news monitoring and less hoarding. Greater self-interest values were associated with less social distancing and more hoarding.
29 June
Maltezou HC, Dedoukou X, Tseroni M, et al. SARS-CoV-2 infection in healthcare personnel with high-risk occupational exposure: evaluation of seven-day exclusion from work policy. Clin Inf Dis June 29, 2020. Full-text: https://doi.org/10.1093/cid/ciaa888
In this study, 3,398 occupationally-exposed HCW were followed prospectively, among them 1,599 (47.1%) with low-risk, 765 (22.5%) with moderate-risk, and 1,031 (30.4%) with high-risk exposures. Of the 66 HCW with COVID-19, 46, 7, and 13 had a history of high-, moderate- or low-risk exposure. Male gender, administrative personnel, underlying disease and high-risk exposure were significantly associated with an increased risk for the onset of COVID-19. HCW with high-risk occupational exposure to COVID-19 had increased probability of serious morbidity, healthcare seeking, hospitalization and absenteeism.
Shrestha NK, Canosa FM, Nowacki AS, et al. Distribution of Transmission Potential during Non-Severe COVID-19 Illness. Clin Inf Dis 29 June 2020. Full-text: https://doi.org/10.1093/cid/ciaa886
Infectivity lasts ten days. In 230 HCW with non-severe COVID-19, viral loads declined by orders of magnitude within a few days of symptom onset. Of the area under the curve (the distribution of transmission potential over time during the course of illness) spanning symptom onset to 30 days, 86.3% lay within the first 5 days, 96.9% within the first 7 days, and 99.7% within the first 10 days. The only variable significantly associated with viral load was time from onset of symptoms.
Pastorino B, Touret F, Gilles M, de Lamballerie X, Charrel RN. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg Infect Dis. 2020 Sep [date cited]. Full-text: https://doi.org/10.3201/eid2609.201788
Clean surfaces! The authors observed a steady infectivity (< 1 log10 drop) on plastic, a 3.5 log10 decrease on glass, and a 6 log10 drop on aluminum within 96 hours. Data showed that SARS-CoV-2 infectivity was remarkably preserved in the presence of proteins (bovine serum albumin), regardless of the type of surface.
27 June
Ortega R, Gonzalez M, Nozari A, et al. Personal Protective Equipment and Covid-19. N Engl J Med 2020; June 25. Full-text: https://doi.org/10.1056/NEJMvcm2014809
Helpful video, demonstrating the complex procedure for putting on and removing PPE that has been recommended by the CDC to minimize the risk of exposure to infectious material during the care of patients with COVID-19.
Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science 26 Jun 2020: Vol. 368, Issue 6498, pp. 1422-1424. Full-text: https://doi.org/10.1126/science.abc6197
Aerosol transmission of viruses must be acknowledged as a key factor leading to the spread of infectious respiratory diseases. This viewpoint summarizes current research that is already leading to a better understanding of the importance of airborne transmission.
25 June
Chou R, Dana R, Jungbauer R, et al. Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings. Ann Int Med 24 Jun 2020. Full-text: https://doi.org/10.7326/M20-3213
According to this “living rapid” review of 39 studies (18 randomized controlled trials and 21 observational studies; 33,867 participants), evidence on mask effectiveness for respiratory infection prevention is stronger in health care than community settings. N95 respirators might reduce SARS-CoV-1 risk versus surgical masks in health care settings, but applicability to SARS-CoV-2 is uncertain.
24 June
Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ Res. 2020 Jun 13;188:109819. PubMed: https://pubmed.gov/32569870. Full-text: https://doi.org/10.1016/j.envres.2020.109819 l (Important)
This review paper “intends to outline the literature” (no doubt they‘ve done it, 139 references!) concerning the transmission of virus-laden droplets and aerosols in different environmental settings. Nice pictures, demonstrating the behavior of droplets and aerosols resulting from a cough-jet of an infected person in various confined spaces.
Rafferty M, Nihtianova S, Amirian ES. COVID-19 Safety Grades for Businesses—A Possible Mitigation Tool. JAMA Health Forum June 22, 2020. Full-text: https://jamanetwork.com/channels/health-forum/fullarticle/2767689
The average customer has no reliable way of knowing whether those in a restaurant kitchen or in employee-only areas are following good hygiene, wearing facial coverings, and observing social distancing. Many jurisdictions are relying on public health recommendations for businesses, which depend on cooperation and are legally unenforceable. The authors propose a tactic that could provide some of the requisite knowledge individuals need to make more informed decisions.
Carraturo F, Del Giudice C, Morelli M, et al. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ Pollut. 2020 Jun 9;265(Pt B):115010. PubMed: https://pubmed.gov/32570023. Full-text: https://doi.org/10.1016/j.envpol.2020.115010
Reviewing the current literature, these authors come to the conclusion that COVID-19 airborne spread via particulates is not a major transmission route. Virus persistance in water, wastewater, and sludge is very low at more than 20 °C.
Wang Y, Wu W, Cheng Z, et al. Super-factors associated with transmission of occupational COVID-2019 infection among healthcare staff in Wuhan , China. J Hosp Infect. 2020 Jun 20:S0195-6701(20)30308-X. PubMed: https://pubmed.gov/32574702. Full-text: https://doi.org/10.1016/j.jhin.2020.06.023
Don’t touch your nose: This cross-sectional study was conducted among 92 frontline members of medical staff. The main factor that contributed to COVID-19 infections was touching the cheek, nose and mouth while working, emphasizing the need to strengthen hand, oral and nasal hygiene practices. Wearing the right type or size of PPE every time as required and following the operation specifications and operation instructions improved self-protection.
22 June
Rickman HM, Rampling T, Shaw K, et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020 Jun 20. PubMed: https://pubmed.gov/32562422. Full-text: https://doi.org/10.1093/cid/ciaa816
A good example for working up a catastrophy, learning from mistakes. Of 435 cases of PCR-positive inpatients in a London hospital, 47 (11%) met the definition for definite hospital acquisition, with a further 19 (4%) probable hospital-acquired. Symptom onset for these 66 hospital acquired cases was a median of 26 days (IQR 13-55) from admission. 24 (36%) patients died. Evidence of patient-to-patient transmission through contact in the same hospital bay was found in 55%.
19 June
Xu XK, Liu XF, Wu Y, et al. Reconstruction of Transmission Pairs for novel Coronavirus Disease 2019 (COVID-19) in mainland China: Estimation of Super-spreading Events, Serial Interval, and Hazard of Infection. Clinical Infectious Diseases 2020. Full-text: https://doi.org/10.1093/cid/ciaa790
The virus is so fast: This database with detailed demographic characteristics, travel history, social relationships, and epidemiological timelines for 1,407 transmission pairs that formed 643 transmission clusters in mainland China used statistical model fittings to identify the super-spreaders and estimate serial interval distributions. There were 34 primary cases identified as super-spreaders, with 5 super-spreading events occurring within households. Serial intervals were short and were estimated as 5.0 (95% CI: 4.4-5.5) and 5.2 (95% CI: 4.9- 5.7) days for household transmissions and 5.2 (95% CI: 4.6-5.8) and 5.3 (95% CrI: 4.9-5.7) days for non-household transmissions, respectively.
Mani NS, Budak JZ, Lan KF, et al. Prevalence of COVID-19 Infection and Outcomes Among Symptomatic Healthcare Workers in Seattle, Washington. Clin Infect Dis. 2020 Jun 16:ciaa761. PubMed: https://pubmed.gov/32548613. Full-text: https://doi.org/10.1093/cid/ciaa761
The authors have stablished two high-throughput employee testing centers in Seattle, Washington with drive-through and walk-through options for symptomatic employees at the University of Washington Medicine system and its affiliated organizations. Between March 12 and April 23, a total of 3,477 symptomatic employees were tested; 185 (5.3%) employees tested positive for COVID-19. The prevalence of SARS-CoV-2 was similar when comparing frontline HCWs (5.2%) to non-frontline staff (5.5%).
18 June
Jing JQ, Liu MJ, Yuan J. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020; (published online June 17.) https://doi.org/10.1016/S1473-3099(20)30471-0
Are children less susceptible? Using a comprehensive contact tracing dataset, the authors estimated secondary attack rate among household contacts to be 12.4% (95% CI 9.8–15.4) when household contacts were defined on the basis of close relatives, and 17.1% (13.3–21.8) when household contacts were defined on the basis of residential address. Compared with the oldest age group (≥ 60 years), the risk of household infection was lower in the youngest age group (< 20 years; odds ratio 0.23) and among adults aged 20–59 years (OR 0.64).
Du W, Yu J, Liu X, Chen H, Lin L, Li Q. Persistence of SARS-CoV-2 virus RNA in feces: A case series of children. J Infect Public Health. 2020 Jun 7. PubMed: https://pubmed.gov/32546439. Full-text: https://doi.org/10.1016/j.jiph.2020.05.025
During follow-up examination after discharge, seven out of ten children contained SARS-CoV-2 virus RNA in their fecal specimens, despite all patients showing negative results in respiratory tract specimens. One out of those seven patients relapsed. The median time from onset to being negative in respiratory tract and fecal specimens was 9 days and 34.43 days, respectively.
Dhand R, Li J. Coughs and Sneezes: Their Role in Transmission of Respiratory Viral Infections, Including SARS-CoV-2. Am J Respir Crit Care Med. 2020 Jun 16. PubMed: https://pubmed.gov/32543913. Full-text: https://doi.org/10.1164/rccm.202004-1263PP l (Important)
Everything you always wanted to know about… coughs and sneezes. A total of 79 references are used to explain how larger droplets produced by coughing and sneezing settle quickly, and the force with which they are expelled determines how far they are dispersed. Nice visuals in the supplement at the end of the paper.
17 June
Steensels D, Oris E, Coninx L, et al. Hospital-Wide SARS-CoV-2 Antibody Screening in 3056 Staff in a Tertiary Center in Belgium. JAMA. 2020 Jun 15. PubMed: https://pubmed.gov/32539107. Full-text: https://doi.org/10.1001/jama.2020.11160
In this large, hospital-wide screening study for SARS-CoV-2 antibodies among hospital staff in a Belgian tertiary care center, neither direct involvement in clinical care nor working in a COVID-19 unit increased the odds of being seropositive, while having a suspected COVID-19 household contact did. Overall, 197 staff (6.4%, 95% CI, 5.5%-7.3%) had IgG antibodies for SARS-CoV-2.
16 June
Cox RJ, Brokstadt KA, Krammer F, et al. Seroconversion in household members of COVID-19 outpatients. Lancet June 15, 2020. Full-text: https://doi.org/10.1016/S1473-3099(20)30466-7
This study from Norway shows that detection of seroconversion might provide a more accurate picture of attack rates in households than intermittent RT-PCR testing. Of 158 cases, 125 (79%) tested positive for antibodies and 12 (8%) were defined as borderline. In 77 household members, 24 (31%) tested positive and two (3%) were borderline.
15 June
Rempel D, Members of the N95DECON Consortium. Scientific Collaboration During the COVID-19 Pandemic: N95DECON.org. Annals of Work Exposures and Health 2020, June 13. Full-text: https://doi.org/10.1093/annweh/wxaa057
This commentary describes the spontaneous formation of an international team of 115 researchers who summarized the literature on safe methods for decontaminating N95 filtering facepiece respirators in response to the supply crisis. The summary reports and fact sheets on the www.n95decon.org website are frequently being updated with new research findings and have had more than 200,000 visits.
12 June
Liu M, Cheng SZ, Xu KW, et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020 Jun 10;369:m2195. PubMed: https://pubmed.gov/32522737. Full-text: https://doi.org/10.1136/bmj.m2195
PPE works well: This study analyzed 420 healthcare professionals (116 doctors and 304 nurses) who were deployed to Wuhan by two affiliated hospitals of Sun Yatsen University and Nanfang Hospital of Southern Medical University for 6-8 weeks from 24 January to 7 April 2020. All were provided with appropriate personal protective equipment to deliver healthcare to patients admitted to hospital with COVID-19. Although all were involved in aerosol generating procedures (high risk of exposure), no-one contracted infection.
Schuit M, Ratnesar-Shumate S, Yolitz J, et al. Airborne SARS-CoV-2 is Rapidly Inactivated by Simulated Sunlight. J Infect Dis. 2020 Jun 11:jiaa334. PubMed: https://pubmed.gov/32525979. Full-text: https://doi.org/10.1093/infdis/jiaa334 l (Important)
Again, it’s sunlight! This study examined the effect of simulated sunlight and relative humidity on the stability of SARS-CoV-2 in aerosols. A 90% loss of virus in simulated saliva was 19 minutes under simulated sunlight levels representative of late winter/early fall, 6 minutes of summer levels and 125 minutes without simulated sunlight across all relative humidity levels. Aerosol transmission of SARS-CoV-2 may be dependent on environmental conditions, particularly sunlight.
11 June
Behrens GM, Cossmann A, Stankov MV. et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection 2020. Full-text: https://www.springermedizin.de/perceived-versus-proven-sars-cov-2-specific-immune-responses-in-/18070162
The gap between perceived risk and evidence: Upon enrollment, HCW in Hannover, Germany, were asked to estimate their personal likelihood of having had a SARS-CoV-2 infection (How high do you rate the probability of having been infected so far? 0–100%). Of 201 study participants, 19% rated their probability greater than 50%. In contrast to the high percentage of self-perceived positive SARS-CoV-2 infection status, only two tested frontline HCPs showed a clearly positive reaction to the ELISA.
10 June
El-Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020 Jun 9. PubMed: https://pubmed.gov/32516833. Full-text: https://doi.org/10.1111/anae.15170
Around 1 in 10 HCW becomes infected: This prospective international multicentre cohort study recruited 1,718 healthcare workers participating in 5,148 tracheal intubation episodes of patients with suspected or confirmed COVID‐19 from 503 hospitals in 17 countries. The overall incidence of the primary endpoint (lab-confirmed COVID-19 diagnosis or new symptoms requiring self-isolation or hospitalisation) was 10.7% over a median follow-up of 32 days. The cumulative incidence within 7, 14 and 21 days of the first tracheal intubation episode was 3.6%, 6.1%, and 8.5%, respectively. The risk varied by country and was higher in females, but was not associated with other factors.
9 June
Matson MJ, Yinda CK, Seifert SN, et al. Effect of Environmental Conditions on SARS-CoV-2 Stability in Human Nasal Mucus and Sputum. Emerg Infect Dis. 2020 Jun 8;26(9). PubMed: https://pubmed.gov/32511089. Full-text: https://wwwnc.cdc.gov/eid/article/26/9/20-2267_article
Environmental conditions affect the stability of the virus in nasal mucus and sputum. The virus is more stable at low temperature and low humidity conditions, whereas warmer temperatures and higher humidity shortened half-life. Although infectious virus was undetectable after 48 hours, viral RNA remained detectable for 7 days.
8 June
Newman A, Smith D, Ghai RR, et al. First Reported Cases of SARS-CoV-2 Infection in Companion Animals – New York, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020 Jun 12;69(23):710-713. PubMed: https://pubmed.gov/32525853. Full-text: https://doi.org/10.15585/mmwr.mm6923e3
American cats are not protected: Two domestic cats with respiratory illnesses lasting 8 and 10 days were the first reported companion animals with SARS-CoV-2 infection in the United States. Both cats were owned by persons with suspected or confirmed COVID-19. According to the authors, persons with COVID-19 should avoid contact with animals. Companion animals that test positive for SARS-CoV-2 should be monitored and separated from persons and other animals until they recover. Good news: both cats fully recovered.
7 June
Han MS, Seong MW, Kim N, et al. Viral RNA Load in Mildly Symptomatic and Asymptomatic Children with COVID-19, Seoul. Emerg Infect Dis. 2020 Jun 4;26(10). PubMed: https://pubmed.gov/32497001. Full-text: https://doi.org/10.3201/eid2610.202449
This study raises doubts on the hypothesis that children are less infectious. Of 12 children (median 6.5 years), 9 were mildly symptomatic and 3 were asymptomatic. Viral RNA load in the nasopharyngeal swabs (and saliva) peaked early at high levels, achieving a median of 7.56 (range 6.19–10.56) log10 copies/mL. Along with positive SARS-CoV-2 RNA in nasopharyngeal swabs, viral RNA was detectable at high concentration for > 3 weeks in fecal samples.
6 June
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020 Jun 1. PubMed: https://pubmed.gov/32497510. Full-text: https://doi.org/10.1016/S0140-6736(20)31142-9 l (Important)
Nothing really new, but this incredible work had to be done. This systematic review identified 172 observational studies across 16 countries and six continents and 44 relevant comparative studies in health-care and non-health-care settings. Transmission of viruses was lower with physical distancing of 1 m or more, compared with a distance of less than 1 m (n=10,736, pooled adjusted odds ratio 0.18), protection was increased as distance was lengthened. Face mask use could result in a large reduction in risk of infection (n=2,647; AOR 0.15), stronger associations with N95 or similar respirators compared with disposable surgical masks or similar. Eye protection also was helpful (n=3,713; AOR 0.22). The findings support face masks, eye protection and physical distancing of 1 m or more.
Iannone P, Castellini G, Coclite D, et al. The need of health policy perspective to protect Healthcare Workers during COVID-19 pandemic. A GRADE rapid review on the N95 respirators effectiveness. PLoS One. 2020 Jun 3. PubMed: https://pubmed.gov/32492045. Full-text: https://doi.org/10.1371/journal.pone.0234025. eCollection 2020
Another review of N95 masks. Four RCTs involving 8,736 HCWs were included. There was no direct high quality evidence on whether N95 respirators are better than surgical masks for HCWs protection from SARS-CoV-2. However, wearing N95 respirators could prevent 73 more clinical respiratory infections per 1000 HCWs compared to surgical masks (low quality evidence).
Fischer RJ, Morris DH, van Doremalen N, et al. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg Infect Dis. 2020 Jun 3;26(9). PubMed: https://pubmed.gov/32491983. Full-text: https://doi.org/10.3201/eid2609.201524
Recycle your masks 2-3 times but not more! Authors have analyzed 4 different decontamination methods – UV light (260–285 nm), 70ºC dry heat, 70% ethanol, and vaporized hydrogen peroxide (VHP), for their ability to reduce contamination with infectious SARS-CoV-2 and their effect on N95 respirator function. UV light inactivated virus rapidly from steel but more slowly on N95 fabric, probably because of its porous nature. Heat caused more rapid inactivation on N95 than on steel; inactivation rates on N95 were comparable to UV. In conclusion, N95 respirators can be decontaminated and reused up to 3 times by using UV light and VHP and 1–2 times by using dry heat. Subsequent rounds of decontamination caused sharp drops in filtration performance.
4 June
Eskew EA, Carlson CJ. Overselling wildlife trade bans will not bolster conservation or pandemic preparedness. Lancet Planetary Health, June 01, 2020. Full-text: https://doi.org/10.1016/S2542-5196(20)30123-6
Nice comment about a wildlife trade ban. Many (including us) have been quick to advocate for complete restriction of commercial trade, particularly in wet markets (like Wuhan) given their potential role as hotspots of cross-species viral transmission. This collective rhetoric suggests that eliminating wildlife trade is a simple, effective defense against zoonotic pandemics. According to the authors, stopping pandemics is not as simple as stopping wildlife trade. The bad news is that even with extensive wildlife trade bans, crippling zoonotic disease burden remains a near certainty.
Baer S, Kim MC, Kim JY. Notice of Retraction: Effectiveness of Surgical and Cotton Masks in Blocking SARS-CoV-2. Annals Int Med 2020, June 2. https://doi.org/10.7326/L20-0745
Come on, guys. “We are retracting our article….we had not fully recognized the concept of limit of detection (LOD) of the in-house RT-PCR used in the study (2.63 log copies/mL), and we regret our failure to express the values below LOD as “<LOD (value).” The LOD is a statistical measure of the lowest quantity of the analyte that can be distinguished from the absence of that analyte. Therefore, values below the LOD are unreliable and our findings are uninterpretable.“ We regret, too.
Baer S, Kim MC, Kim JY, et al. Effectiveness of Surgical and Cotton Masks in Blocking SARS-CoV-2: A Controlled Comparison in 4 Patients. Ann Intern Med. 2020 Apr 6. PubMed: https://pubmed.gov/32251511. Full-text: https://doi.org/10.7326/M20-1342
This was our comment on this study in April: “Very small study, but both surgical and cotton masks appeared to be ineffective in preventing the virus dissemination from the coughs of patients with COVID-19 to the environment and external mask surface.”
Nardell EA, Nathavitharana RR. Airborne Spread of SARS-CoV-2 and a Potential Role for Air Disinfection. JAMA. 2020 Jun 1. PubMed: https://pubmed.gov/32478797. Full-text: https://doi.org/10.1001/jama.2020.7603
We will have to deal with upper-room germicidal UV filters (GUV). According to the authors, preparation for future respiratory viral pathogens should include consideration of the use of upper-room GUV to help mitigate airborne transmission. Sounds complicated, expensive. Bad news.
1 June
Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med. 2020 May 27. PubMed: https://pubmed.gov/32473123. Full-text: https://doi.org/10.1016/S2213-2600(20)30245-9 ll (Outstanding)
Doors and windows open! Important study, analyzing droplet production due to coughs and speech by measuring the droplet size distribution, travel distance and velocity, and the airborne time in relation to the level of air ventilation (no ventilation, mechanical ventilation only, and mechanical ventilation supported by the opening of an entrance door and a small window). In the best ventilated room, after 30 s the number of droplets had halved, whereas with no ventilation this took about 5 min!
Tam PCK, Ly KM, Kernich ML, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020 May 30:ciaa673. PubMed: https://pubmed.gov/32472683. Full-text: https://doi.org/10.1093/cid/ciaa673
A second case of detectable SARS-CoV-2 RNA from human milk in a patient with COVID-19. Despite mild clinical symptoms, the patient had detectable virus in human milk in two separate samples taken ten days apart but interspersed with a number of negative results.
31 May
Chan JF, Yuan S, Zhang AJ, et al. Surgical mask partition reduces the risk of non-contact transmission in a golden Syrian hamster model for Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2020 May 30. PubMed: https://pubmed.gov/32472679. Full-text: https://doi.org/10.1093/cid/ciaa644
They work! Even in a hamster model. Surgical mask partition for challenged index or naïve hamsters significantly reduced transmission.
29 May
On Kwok K, Hin Chan HH, Huang Y, et al. Inferring super-spreading from transmission clusters of COVID-19 in Hong Kong, Japan and Singapore. J Hosp Infect. 2020 May 21:S0195-6701(20)30258-9. PubMed: https://pubmed.gov/32446721. Full-text: https://doi.org/10.1016/j.jhin.2020.05.027 l (Important)
Super-spreading events in an outbreak can change the nature of an epidemic. Therefore, it is useful for public health teams to determine if an ongoing outbreak has any contribution from such events, which may be amenable to interventions. The dispersion factor (k) from empirical data on clusters of epidemiologically-linked COVID-19 cases in Hong Kong, Japan and Singapore was relatively high, indicating that large cluster sizes, compatible with super-spreading, were unlikely.
28 May
Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science. 2020 Jun 26;368(6498):1422-1424. PubMed: https://pubmed.gov/32461212. Full-text: https://doi.org/10.1126/science.abc6197
This perspective clearly shows that masks and testing are necessary and essential to combat asymptomatic spread in aerosols and droplets. It cannot be repeated often enough: infectious aerosol particles can be released during breathing and speaking by asymptomatic infected individuals. No masking maximizes exposure, whereas universal masking results in the least exposure.
Zhang Y, Li Y, Wang L, Li M, Zhou X. Evaluating Transmission Heterogeneity and Super-Spreading Event of COVID-19 in a Metropolis of China. Int J Environ Res Public Health. 2020 May 24;17(10):E3705. PubMed: https://pubmed.gov/32456346. Full-text: https://doi.org/10.3390/ijerph17103705
Over the last few weeks, it has become very clear that some individuals spread to a disproportionate number of individuals, compared to most individuals who infect a few or no-one. This important paper looked at transmission heterogeneity and the emergence of these super-spreading events (SSEs). In total, 135 cases from official public sources in Tianjin, China were grouped into 43 transmission chains. The reproductive number R and the dispersion parameter k (lower value indicating higher heterogeneity) were estimated to be 0.67 (95% CI: 0.54-0.84) and 0.25 (95% CI: 0.13-0.88), respectively. Transmission was very heterogeneous and one SSE was identified. Transmission characteristics of COVID-19 need more exploration and investigation on a large scale.
Zhang W, Cheng W, Luo L, et al. Secondary Transmission of Coronavirus Disease from Presymptomatic Persons, China. Emerg Infect Dis. 2020 May 26;26(8). PubMed: https://pubmed.gov/32453686. Full-text: https://doi.org/10.3201/eid2608.201142
Contact-tracing surveillance data collected in Guangzhou, China during January 28 – March 15, 2020, to explore the secondary attack rate from different types of contact with 38 pre-symptomatic patients. The secondary attack rates (SAR) among household contacts was 16.1% and was 1.1% for social contacts, and 0 for workplace contacts. Older close contacts had the highest SAR compared to other age groups.
27 May
Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell, May 26, 2020. Full-text: https://doi.org/10.1016/j.cell.2020.05.042
This study quantitated differences in ACE2 receptor expression and SARS-CoV-2 infectivity in the nose (high) vs the peripheral lung (low). If the nasal cavity is the initial site mediating seeding of the lung via aspiration, these studies argue for the widespread use of masks to prevent aerosol, large droplet, and/or mechanical exposure to the nasal passages.
26 May
Nordling L. Study tells ‘remarkable story’ about COVID-19’s deadly rampage through a South African hospital. May 25, 2020. Full-text: https://www.sciencemag.org/news/2020/05/study-tells-remarkable-story-about-covid-19-s-deadly-rampage-through-south-african
Screen the staff! Incredible story about a man who sought help for coronavirus symptoms on March 9, spending only a few hours at the emergency department of a hospital in Durban, South Africa. He was kept separate in a triage area, but that room was reached through the main resuscitation bay, where a stroke patient occupied a bed. Both patients were seen by the same doctor. After likely transmitting the virus to the stroke patient, the results, eight weeks later were: 39 patients and 80 staff linked to the hospital had been infected, and 15/39 patients had died. Lesson learnt: Nosocomial outbreaks may be a major amplifier of COVID-19 transmission.
Groß R, Conzelmann C, Müller JA, et al. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020 May 21:S0140-6736(20)31181-8. PubMed: https://pubmed.gov/32446324. Full-text: https://doi.org/10.1016/S0140-6736(20)31181-8
SARSCoV2 RNA in milk samples from an infected mother (two mothers were examined) was found on 4 consecutive days. Detection of viral RNA in milk coincided with mild COVID19 symptoms and a SARSCoV2 positive diagnostic test of the newborn.
24 May
Marjolein F. Q. Kluytmans-van den Bergh MF, Buiting AG, Pas SD, et al. Prevalence and Clinical Presentation of Health Care Workers With Symptoms of Coronavirus Disease 2019 in 2 Dutch Hospitals During an Early Phase of the Pandemic. JAMA Netw Open. 2020;3(5):e209673. https://doi.org/10.1001/jamanetworkopen.2020.9673
Of 9,705 HCWs from the Netherlands, 1353 (14%) reported fever or respiratory symptoms and were tested. Of those, 86 HCWs (6%) were infected. Hospital acquisition was unlikely to explain the vast majority of cases. Of note, 54 HCWs (63%) mentioned having worked while being symptomatic.
Lai X, Wang M, Quin C, et al. Coronavirus Disease 2019 (COVID-2019) Infection Among Health Care Workers and Implications for Prevention Measures in a Tertiary Hospital in Wuhan, China. JAMA Netw Open May 21, 2020;3(5):e209666. Full-text: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2766227
Overall, 110 of 9,684 HCWs in Tongji Hospital tested positive, with an infection rate of 1.1%. Most infections occurred at the early stage of the epidemic (before January 25), before protective measures were taken. Of those who worked in fever clinics or wards, 17/3110 were infected, indicating an infection rate of 0.5% among first-line HCWs. Of note, a higher rate of infection was found in non-first-line HCW (93/6.574, 1.4%). Authors speculate that this was due to insufficient protective measures available in clinical departments other than fever clinics and wards.
Clase CM, Fu EL, Joseph M, et al. Cloth Masks May Prevent Transmission of COVID-19: An Evidence-Based, Risk-Based Approach. Ann Int Med 2020, May 22. Full-text: https://www.acpjournals.org/doi/10.7326/M20-2567
According to the authors, there is high-quality, consistent evidence that many (but not all) cloth masks reduce droplet and aerosol transmission and may be effective in reducing contamination of the environment. No direct evidence indicates that public mask wearing protects either the wearer or others. However, the possible benefit of a modest reduction in transmission likely outweighs the possibility of harm.
23 May
Bao L, Gao H, Deng W, et al. Transmission of SARS-CoV-2 via close contact and respiratory droplets among hACE2 mice. J Inf Dis 2020, May 23. Full-text: https://doi.org/10.1093/infdis/jiaa281
Using ACE2 mice, authors simulated different transmission modes. Close contact and droplets worked better than aerosol exposition. Animals could not be experimentally infected via aerosol inoculation until continuous exposition for up to 25 min even with high virus concentrations.
22 May
Guasp M, Laredo C, Urra X. Higher solar irradiance is associated with a lower incidence of COVID-19. Clin Infect Dis. 2020 May 19:ciaa575. PubMed: https://pubmed.gov/32426805. Full-text: https://doi.org/10.1093/cid/ciaa575
Increasing sunlight exposure in the upcoming weeks may help flatten the curve! UVB radiation from sunlight (the primary source of UV radiation) is the principal environmentally effective virucide, probably much more relevant than temperature and humidity. Authors studied the relationship between the incidence of COVID-19, demographic, and climatologic measurements in different regions across the world. They show a significant association of the incidence of COVID-19 and both reduced solar irradiance and increased population density, highlighting the sterilizing properties of UV radiation.
Ratnesar-Shumate S, Williams G, Green B, et al. Simulated Sunlight Rapidly Inactivates SARS-CoV-2 on Surfaces. J Infect Dis. 2020 May 20:jiaa274. PubMed: https://pubmed.gov/32432672. Full-text: https://doi.org/10.1093/infdis/jiaa274 l (Important)
This lab data supports the above observation of inactivation. Store your masks in the sun! Simulated sunlight rapidly inactivated SARS-CoV-2 suspended in either simulated saliva or culture media and dried on stainless steel plates. Ninety percent of infectious virus was inactivated every 6.8 minutes in simulated saliva and every 14.3 minutes in culture media when exposed to simulated sunlight representative of the sun on a clear summer day. No significant decay was observed in darkness over 60 minutes.
Bunyavanich S, Do A, Vicencio A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020 May 20. PubMed: https://pubmed.gov/32432657. Full-text: https://doi.org/10.1001/jama.2020.8707
Is this the reason for lower infection rates in children? As the nasal epithelium is one of the first sites of infection, investigators evaluated the expression of ACE in nasal epithelial samples collected 2015-2018 as part of an asthma study. Among a cohort of 305 patients, all age groups had higher expression of ACE2 in the nasal epithelium compared with younger children (4-9 years old). ACE2 expression was age-dependent and higher with each subsequent age group after adjusting for sex and asthma. A good argument for opening a day care center for children.
20 May
Ortega R, Gonzalez M, Nozari A, et al. Personal Protective Equipment and Covid-19. NEJM 2020, May 19. Full-text: https://doi.org/1056/NEJMvcm2014809
Prevention works only through training and demonstrated competency in putting on and removing personal protective equipment (PPE). This video demonstrates a procedure for “donning and doffing“ PPE.
James A, Eagle L, Phillips C. High COVID-19 Attack Rate Among Attendees at Events at a Church — Arkansas, March 2020. MMWR 2020, May 19. Full-text: http://dx.doi.org/10.15585/mmwr.mm6920e2
High transmission rates of SARS-CoV-2 have been reported from hospitals, long-term care facilities, family gatherings, choir practice. This report describes church events. In total, 35 confirmed COVID-19 cases occurred among 92 attendees at church events during March 6–11; estimated attack rates ranged from 38% to 78%. Of note, a higher proportion of adults aged 19–64 years and ≥65 years received positive test results than did younger persons.
Hastie CE, Mackay DF, Ho F, et al. Vitamin D Concentrations and COVID-19 Infection in UK Biobank. Diabetes Metab Syndr 2020 May 7;14(4):561-565. Full-text: https://doi.org/10.1016/j.dsx.2020.04.050
No link between vitamin D and infection risk. Of 348,598 UK biobank participants, 449 had confirmed COVID-19 infection. Ethnicity was associated with COVID-19 infection (blacks versus whites OR = 5.32, South Asians versus whites OR = 2.65). Vitamin D was not associated with COVID-19 infection, after adjustment for confounders. Vitamin D did not explain ethnic differences.
18 May
Sit TH, Brackman CJ, Ip SM et al. Infection of dogs with SARS-CoV-2. Nature 2020. https://doi.org/10.1038/s41586-020-2334-5. Full-text: https://www.nature.com/articles/s41586-020-2334-5#citeas
Two out of fifteen dogs (one Pomeranian and one German Shepherd) from households with confirmed human cases of COVID-19 in Hong Kong were found to be infected. Both dogs remained asymptomatic but later developed antibody responses detected using plaque reduction neutralization assays. Genetic analysis suggested that the dogs caught the virus from their owners. It still remains unclear whether infected dogs can transmit the virus to other animals or back to humans.
Seyer A, Sanlidag T. Solar ultraviolet radiation sensitivity of SARS-CoV-2. Lancet Microbe 2020, 1:e8-e9, May, 2020. Full-text: https://doi.org/10.1016/S2666-5247(20)30013-6
Sunlight reaching the ground lacks germicidal ultraviolet C radiation. According to the authors, scientists should avoid voicing assumptions on the effect of sunlight on viral transmission.
17 May
Böhmer MM, Buchholz U, Corman VM. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis 2020, May 15. Full-text: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30314-5/fulltext
The German patient zero was a Chinese person who visited Germany for professional reasons. Sixteen persons became infected. This thorough description of transmission dynamics revealed that attack rates were 75% among members of a household cluster in common isolation, 10% among household contacts only together until isolation of case, and 5% among non-household high-risk contacts. Although most patients presented with only mild and non-specific symptoms, infectiousness before or on the day of symptom onset was substantial. Additionally, the incubation period was often very short and false-negative tests occurred.
Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice – Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020 May 15;69(19):606-610. PubMed: https://pubmed.gov/32407303. Full-text: https://doi.org/10.15585/mmwr.mm6919e6 l (Important)
What a disaster! Among 61 persons who attended a March 10 choir practice, 32 confirmed and 20 probable secondary COVID-19 cases occurred. Three were hospitalized (5.7%), and two died (3.7%). The 2.5 hour singing practice provided several opportunities for droplet and fomite transmission, including members sitting close to one another, sharing snacks, and stacking chairs at the end of the practice. Chairs were arranged in six rows of 20 chairs each, spaced 6–10 inches apart with a center aisle dividing left and right stages. Most choir members sat in their usual rehearsal seats (see full paper for more details). The act of singing itself might have contributed to transmission through emission of aerosols, which is affected by the loudness of vocalization.
16 May
Zheng L, Wang X, Zhou C, et al. Analysis of the infection status of the health care workers in Wuhan during the COVID-19 outbreak: A cross-sectional study. Clinical Infectious Diseases 2020, May 15. Full-text: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa588/5837357
By now, the most comprehensive data on infections among HCW. Among 2,457 infected HCW in Wuhan, China, 52% were nurses, 34% were doctors and 14% were medical staff. Case infection rate of nurses (2.22%) was higher than that of doctors (1.92%). The majority (89%) came from general hospitals. The case infection rate of HCW (2.10%) was dramatically higher than that of non-HCW (0.43%). The case fatality rate of was significantly lower (0.69% versus 5.30%).
14 May
Halfmann PJ, Hatta M, Chiba S, et al. Transmission of SARS-CoV-2 in Domestic Cats. N Engl J Med. 2020 May 13. PubMed: https://pubmed.gov/32402157. Full-text: https://doi.org/10.1056/NEJMc2013400
Three domestic cats were inoculated with SARS-CoV-2. One day later, an uninfected cat was co-housed with each of the inoculated cats. All six cats became infected but none showed any symptoms. All cats had developed antibody titers on day 24. Are cats potential intermediate hosts in chains of human–cat–human transmission?
Hamiel U, Kozer E, Youngster I. SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults. JAMA. 2020 May 13. PubMed: https://pubmed.gov/32401274. Full-text: https://doi.org/10.1001/jama.2020.8189
Could it be possible that a BCG vaccination is protective? No. In this very large cohort of Israeli adults aged 35 to 41 years, BCG vaccination in childhood was associated with a very similar rate of positive test results for SARS-CoV-2 compared with no vaccination.
13 May
Wu J, Huang Y, Tu C, et al. Household Transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. 2020 May 11. PubMed: https://pubmed.gov/32392331. Full-text: https://doi.org/10.1093/cid/ciaa557
The next study on a relatively low transmission rate among household contacts. A total of 35 index cases from Zhuhai, China and their 148 household contacts were carefully analyzed, using questionnaires, active symptom monitoring and nasopharyngeal swabs. The second infection rate in the household context was 32% (95% CI 22-44%). Multivariate analysis showed that household contacts with underlying medical conditions, a history of direct exposure to Wuhan, and shared vehicle with an index patient were associated with higher susceptibility.
Hijnen D, Marzano AV, Eyerich K, et al. SARS-CoV-2 Transmission from Presymptomatic Meeting Attendee, Germany. Emerg Infect Dis. 2020 May 11;26(8). PubMed: https://pubmed.gov/32392125. Full-text: https://doi.org/10.3201/eid2608.201235 l (Important)
Wanna make sure that SARS-CoV-2 is transmitted with almost 100% efficacy? Then use advisory boards or comparable settings: Eight dermatologists and 6 scientists from the same company attended a meeting at a hotel in Munich. The meeting was held in a room (≈70 m2) with conventional radiators; a U-shaped set-up of tables were separated by a central aisle >1 m wide. Refreshments were served buffet style in the same room 4 times during the day. After the 9.5 hours of discussions, participants had dinner in a nearby restaurant. Additional direct contacts were handshakes during welcome and farewells with a few short hugs without kisses and a 45 min. taxi ride with 3 participants. Results: The asymptomatic (!) index patient managed to infect at least 11/13 (!) participants. Note: The meeting was held on February 20; the country had <20 diagnosed cases at the time.
7 May
Liao L, Xiao W, Zhao M, et al. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano. 2020 May 5. PubMed: https://pubmed.gov/32368894. Full-text: https://doi.org/10.1021/acsnano.0c03597
How can we re-use N95 respirators? Heat is better than sun or vapors. At 85°C, 50 cycles of heat treatment did not significantly change filtration efficiency. At low humidity and temperatures up to 100 degrees, 20 cycles were possible. Ultraviolet irradiation was a secondary choice, which was able to withstand 10 cycles of treatment and showed small degradation by 20 cycles. However, UV can potentially impact the material strength. Treatments involving liquids and vapors require caution, as steam, alcohol, and household bleach all may lead to degradation of the filtration efficiency.
29 April
Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 Apr 27. PubMed: https://pubmed.gov/32340022. Full-text: https://doi.org/10.1038/s41586-020-2271-3 l (Important)
Toilets are the hot spots! Important study, sampling airborne SARS-CoV-2 and its aerosol deposition at 30 sites in two designated hospitals and public areas in Wuhan in February/March. The concentration in isolation wards and ventilated patient rooms was very low, but it was elevated in the patients’ toilet areas. Levels were undetectable in the majority of public areas outside the hospitals and was undetectable except in two areas prone to crowding. Room ventilation, open space, sanitization of protective apparel as well as proper use and disinfection of toilet areas can effectively limit the concentration of SARS-CoV-2 RNA in aerosols.
Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020 Apr 27. PubMed: https://pubmed.gov/32339382. Full-text: https://doi.org/10.1111/1471-0528.16278
Vaginal delivery is associated with low risk of intrapartum infection. Of 42 women with COVID-19 (19 with pneumonia), 24 delivered vaginally. Only 1/24 new-born had a positive test. Two women transmitted the virus while breastfeeding without a mask.
27 April
Schwierzeck V, König JC, Kühn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clinical Infectious Diseases, 27 April 2020. Full-text: https://doi.org/10.1093/cid/ciaa491
The next outbreak, occurring in a pediatric dialysis unit in Münster, Germany, comprising a total of 12 cases. After careful investigation, the authors found that none of 32 persons with type II exposure became infected (= shared indoor environment without cumulative 15 minutes face-to-face contact, HCWs exposed during treatment or nursing in a distance of > 2 meters, without appropriate personal protective equipment such as surgical masks, etc.).
Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity. Lancet. 2020 Apr 25;395(10233):1339-1340. PubMed: https://pubmed.gov/32304647. Full-text: https://doi.org/10.1016/S0140-6736(20)30868-0
Several reports from China have suggested prolonged shedding of the virus by measuring viral RNA in different body fluids. The authors emphasize an important issue in the current discussion. The presence of nucleic acid alone cannot be used to define viral shedding or infection potential. For many viral diseases including SARS-CoV or MERS-CoV, it is well known that viral RNA can be detected long after the disappearance of infectious virus.
26 April
Canova V, Lederer Schlapfer H, Piso RJ, et al. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020 Apr 25;150:w20257. PubMed: https://pubmed.gov/32333603. Full-text: https://doi.org/Swiss Med Wkly. 2020;150:w20257
Good news. Among 21 healthcare workers who had contact with an initially undiagnosed COVID-19 case, transmission risk was low, especially during short contacts.
25 April
Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020 Apr 24. PubMed: https://pubmed.gov/32329971. Full-text: https://doi.org/10.1056/NEJMoa2008457
The next outbreak in King County, Washington: a skilled nursing facility facing rapid and widespread transmission of the virus, leading to 17 deaths in 57 residents. Of note, 27/48 with positive test results were asymptomatic at the time of testing and most likely contributed to transmission. Infection-control strategies focussing solely on symptomatic residents are not sufficient! Test them all, immediately!
Tobias A, Molina T. Is temperature reducing the transmission of COVID-19 ? Environ Res. 2020 Apr 18;186:109553. PubMed: https://pubmed.gov/32330766. Full-text: https://doi.org/10.1016/j.envres.2020.109553
Measuring temperature’s impact on transmission rates is almost impossible in such a dynamic pandemic. The authors have made a heroic attempt, showing that the number of diagnosed cases may increase below a maximum temperature of 10° C and linearly decreasing afterward. Thus, the arrival of summer could reduce the transmission of the COVID-19. However, this is only a first clue that has to be confirmed.
24 April
Park SY, Kim YM, Yi S, et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg Infect Dis. 2020 Apr 23;26(8). PubMed: https://pubmed.gov/32324530. Full-text: https://doi.org/10.3201/eid2608.201274
Epidemiologic characteristics of a COVID-19 outbreak centered in a call center in South Korea, indicating an attack rate of 8.5% within the whole building. If results were restricted to one floor, the attack rate was as high as 43.5%. Among the 97 confirmed case-patients, 92% were symptomatic at the time of investigation and 4% were presymptomatic. Only 4% remained asymptomatic after 14 days of isolation.
Keshtkar-Jahromi M, Sulkowski M, Holakouie-Naieni K. Public Masking: An Urgent Need to Revise Global Policies to Protect against Novel Coronavirus Disease (COVID-19). Am J Trop Med Hyg. 2020 Apr 22. PubMed: https://pubmed.gov/32323645. Full-text: https://doi.org/10.4269/ajtmh.20-0305
Brief review. The authors highly recommend mass masking around the world during the pandemic. Whereas surgical masks are the preferred recommendation for the general public, cloth masks should be considered as a substitute if supplies are limited or surgical masks are not available.
