The Highlights
Saunders KO, Lee E, Parks R, et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021 May 10. PubMed: https://pubmed.gov/33971664. Full-text: https://doi.org/10.1038/s41586-021-03594-0
Vaccination of macaques with a multimeric pan-coronavirus mRNA nanoparticle vaccine elicited cross-neutralizing antibody responses against various viral strains (batCoVs, SARS-CoV-1, SARS-CoV-2, as well as SARS-CoV-2 variants B.1.1.7, P.1, and B.1.351), protecting the animals against SARS-CoV-2 in upper and lower respiratory tracts (Saunders 2021).
Pezzotti P, Fabiani M, Urdiales AM, et al. Impatto della vaccinazione COVID-19 sul rischio di infezione da SARS-CoV-2 e successivo ricovero e decesso in Italia (27.12.2020 – 03.05.2021). Istituto Superiore di Sanità (Italia) 2021, published 15 May. Full text: https://www.epicentro.iss.it/vaccini/covid-19-report-valutazione-vaccinazione
Two weeks after administration of the first COVID-19 vaccine dose, the risk of SARS-CoV-2 infection, hospitalization and death progressively decreases up to about 35 days, both in men and women and in people of different age groups. These are the results of a study by the Italian National Institute of Health (Istituto Superiore di Sanità, ISS) which analyzed 7,370,008 individuals vaccinated as of 4 April 2021. 65% of the study population had received the first injection the BioNTech/Pfizer vaccine, 6% the first Moderna vaccine and 29 the first AstraZeneca injection (Pezzotti 2021) [1]. The authors describe a
-
- ~80% reduction for the risk of receiving a diagnosis of SARS-CoV-2 infection
- 90% reduction for the risk of hospitalization
- 95% reduction for the risk of death (see Figure xxx)
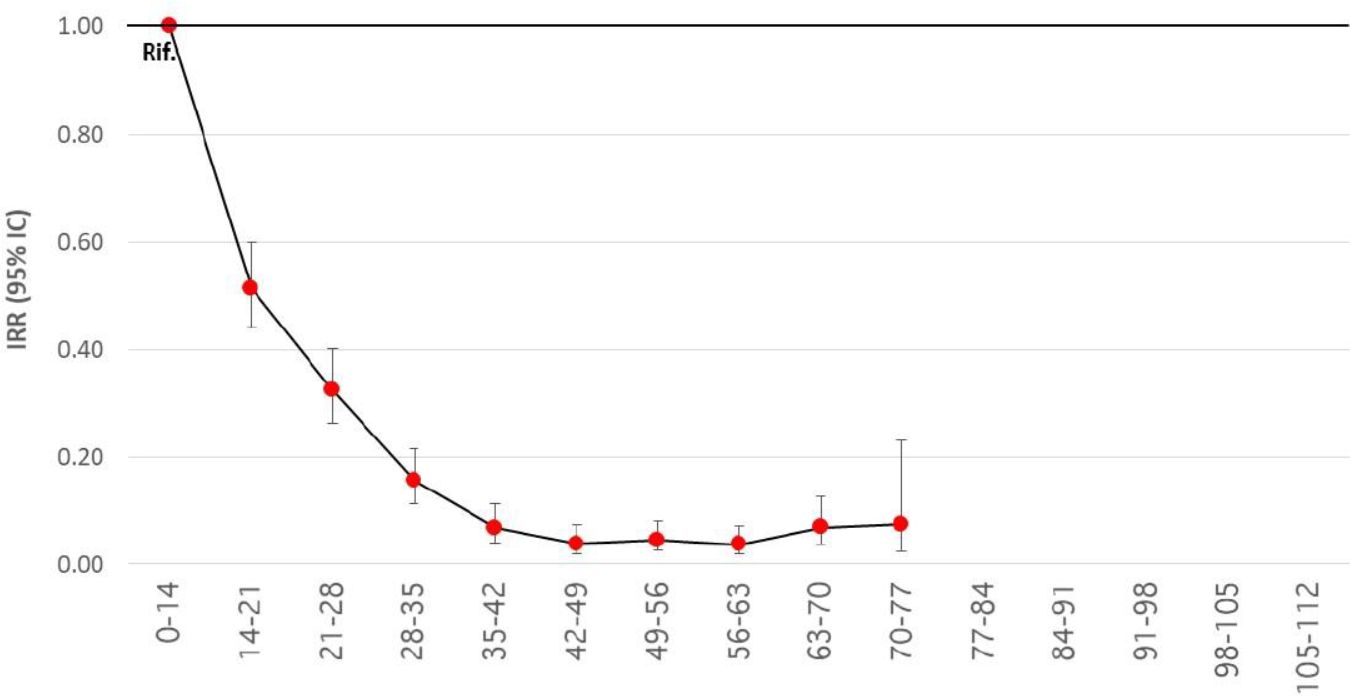
Figure xxx. Reduction of the risk of diagnosis and subsequent death at different time intervals from administration of any first dose of the BioNTech/Pfizer, Moderna or AstraZeneca vaccine, starting from the beginning of the vaccination cycle compared to the period 0-14 days from the first dose (reference period).
[1] The vast majority of mRNA vaccinees received two doses within the usual vaccination schedule (BioNTech/Pfizer: 21 + 4 days, Moderna: 28 + 2). Of those receiving the AstraZeneca vaccine none received the second dose.
13 May
In a small cohort study, 30 pregnant and 16 lactating women developed both humoral and cellular immune responses after vaccination with the BioNTech/Pfizer or the Moderna vaccine. Vaccine-elicited antibodies were also found in infant cord blood and breast milk (Collier 2021).
Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA Network 2021, published 13 May. Full text: https://jamanetwork.com/journals/jama/fullarticle/2780202
12 May
Two shots, two vaccines
The Com-COV trial compares the four possible prime-boost combinations of the BioNTech/Pfizer vaccine and the AstraZeneca vaccine. The preliminary reactogenicity show that among the participants who received the boost vaccine dose 28 days after the first dose, both heterologous vaccine schedules (BioNTech/Pfizer + AstraZeneca or AstraZeneca + BioNTech/Pfizer) induced greater systemic reactogenicity following the boost dose than homologous schedules (BioNTech/Pfizer + BioNTech/Pfizer or AstraZeneca + AstraZeneca); this was accompanied by more frequent use of paracetamol (see Table X) (Shaw 2021). Most of this increase in reactogenicity was observed in the 48 h after the second dose. The authors of the study suggest that routine prophylactic use of paracetamol could help mitigate these effects. They also note that the participants in this trial were aged 50 years and older and that reactogenicity could be higher in younger individuals. Data about the primary immunological outcome are expected in June.
|
||||
|
Feverishness | Paracetamol use | ||
| BioNTech/Pfizer + BioNTech/Pfizer | 118/117 | 21% | 41% | |
| AstraZeneca + AstraZeneca | 115/112 | 10% | 36% | |
| AstraZeneca + BioNTech/Pfizer | 114/110 | 34% | 57% | |
| BioNTech/Pfizer + AstraZeneca | 115/114 | 41% | 60% | |
* Defined as a self-reported feeling of feverishness. Similar increases were observed for chills, fatigue, malaise, headache, joint and muscle ache.
Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. The Lancet 2021, published 12 May. Full text: https://doi.org/10.1016/S0140-6736(21)01115-6
10 May
Upgraded: B.1.617.2
The variant B.1.617.2 (a sub-lineage of B.1.617 first detected in [fdi] Indi) has been upgraded by WHO to a variant of concerns (on par with B.1.1.7 fdi England, B.1.351 fdi South Africa and P.1 fdi Brazil) (NYTimes 20210510). The variant, also designated as VOC21APR-02, seems to be as highly transmissible as B.1.1.7. In the UK, 509 cases had been genomically confirmed by May 10, both imported (n=157) and domestically-acquired (Public Health England 20210507). In a small in vitro study, B.1.617 evaded antibodies induced by infection (15 ICU COVID-19 patients) or vaccination (15 recipients of the BioNTech/Pfizer vaccine), although with moderate efficiency (Hoffmann 2021). In another study, samples from convalescent patients and all samples from individuals vaccinated with mRNA vaccines (BioNTech/Pfizer or Moderna) still had neutralizing activity against B.1.617.1 (although the variant was 7 times more resistant to neutralization) (Edara 2021). Antibody evasion of B.1.617 might contribute to the rapid spread of B.1.617.
- Public Health England 20210507. SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 10. UK Government 2021, updated 7 May. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf – accessed 11 May 2021.
- Hoffmann M, Hofmann-Winkler H, Krüger N, et al. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination. bioRxiv 2021, posted 5 May. Full text: https://doi.org/10.1101/2021.05.04.442663
- Edara VV, Lai L, Sahoo M, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv 2021, posted 10 May. Full text: https://doi.org/10.1101/2021.05.09.443299
The FDA authorizes the BioNTech/Pfizer vaccine for Children 12 to 15. The FDA has lowered the age that people can receive the BioNTech/Pfizer vaccine to 12 (FDA 20210510). The vaccine is expected to be available for children 12 to 15 years of age as early as this week. This expansion of the emergency use authorization (EUA) will allow middle school-aged students to get vaccinated before the beginning of the next school year.
