Home | Daily Science: TOP 10 | TOP 10 BOOK (PDF)
By Christian Hoffmann &
Bernd S. Kamps
17 October
Transmission
Atrubin D, Wiese M, Bohinc B. An Outbreak of COVID-19 Associated with a Recreational Hockey Game — Florida, June 2020. Full-text: http://dx.doi.org/10.15585/mmwr.mm6941a4
On June 16, 2020, a recreational ice hockey game was played between two teams, each consisting of 11 players (typically six on the ice and five on the bench at any given time). The players were men aged 19–53 years. During the 5 days after the game, 15 persons (14 of the 22 players and a rink staff member) experienced signs and symptoms compatible with coronavirus disease 2019 (COVID-19).
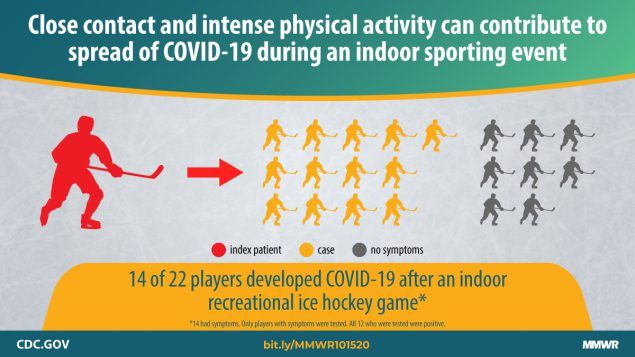
Copyright CDC.gov. Reproduced with permission.
Do you remember our September 12 Top 10? In an unintentional experiment, the German national team of amateur boxers proved that you can achieve a 100% transmission rate in a small group within days. In a training camp, some of the 18 athletes and 7 coaches and supervisors had cold symptoms four days prior. All 25 persons tested positive for SARS-CoV-2. If you read German, read Anonymous. Deutsche Box-Olympiamannschaft mit Coronavirus infiziert. Die Zeit 2020, published 12 September. Full-text: https://www.zeit.de/sport/2020-09/trainingslager-oesterreich-deutsche-box-olympiamannschaft-coronavirus-infektion-quarantaene
Immunology
Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Dis 2020, pubished 16 October. Full-text: https://doi.org/10.1016/S2213-2600(20)30404-5
Daniel Leisman and colleagues question the role of a cytokine storm in COVID-19-induced organ dysfunction after a systematic review and meta-analysis of 25 COVID-19 studies (n = 1245 patients) and four trials each in sepsis (n = 5320), cytokine release syndrome (n = 72), and acute respiratory distress syndrome unrelated to COVID-19 (n = 2767). Mean interleukin-6 concentrations were nearly 100 times higher in patients with cytokine release syndrome (3110.5 pg/mL), 27 times higher in patients with sepsis (983.6 pg/mL), and 12 times higher in patients with acute respiratory distress syndrome unrelated to COVID-19 (460 pg/mL). The authors conclude that alternative mechanisms of COVID-19-induced organ dysfunction are worth considering and that immune-activating treatments (i.e., interferons, IL-7, or checkpoint inhibition) might merit investigation.
Lee S, Channappanavar R, Kanneganti TD. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol 2020, published 15 October. Full-text: https://doi.org/10.1016/j.it.2020.10.005
The authors develop the current understanding of innate immune responses, inflammasome activation, inflammatory cell death pathways, and cytokine secretion during SARS-CoV, MERS-CoV, and SARS-CoV-2 infection. Your Sunday morning review.
Vaccine
Xia S, Zhang Y, Wang Y, e al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2020, published 15 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30831-8
A Chinese candidate vaccine, BBIBP-CorV (Beijing Institute of Biological Products), based on inactivated coronavirus, seems to be safe and elicits an antibody response. This is the first study of an inactivated SARS-CoV-2 vaccine to include participants older than 60 years. In these participants, antibodies took up to 42 days to be detected, compared with 28 days for participants aged 18 to 59. As expected, antibody levels were lower in those aged 60 to 80 years. Two-dose immunization with 4 μg vaccine on days 0 and 21 or days 0 and 28 achieved higher neutralizing antibody titers than the single 8 μg dose or 4 μg dose on days 0 and 14. A Phase III trial of BBIBP-CorV is currently underway in Abu Dhabi and the United Arab Emirates.
See also the comment by Isakova-Sivak I, Rudenko L. A promising inactivated whole-virion SARS-CoV-2 vaccine. Lancet Infect Dis 2020, published 15 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30832-X
Krause PR, Grubner MF. Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. N Engl J Med 2020, published 16 October. Full-text: https://doi.org/10.1056/NEJMp2031373
There should be no emergency use authorization (EUA) of any COVID-19 vaccine without a median follow-up duration of at least 2 months after completion of the full phase 3 vaccination regimen. Normally, the FDA requires at least 6 months of safety follow-up for serious and other medically attended adverse events in a sufficient number of vaccinees. Philip Krause and Marion Gruber warn that any curtailment of this minimum follow-up could destroy the scientific credibility for future vaccines in the United States. Also see FDA’s Vaccines and Related Biological Products Committee Open Hearing, 22 Oct 2020, https://www.youtube.com/watch?v=1XTiL9rUpkg&feature=youtu.be.
Kurup D, Wirblich C, Ramage H, et al. Rabies virus-based COVID-19 vaccine CORAVAX™ induces high levels of neutralizing antibodies against SARS-CoV-2. npj Vaccines 5, 98 (2020). Full-text: https://doi.org/10.1038/s41541-020-00248-6
The authors show the rapid development of a novel, efficient, and safe COVID-19 vaccine using a rabies virus-based vector. Both a live and an inactivated rabies virus containing the SARS-CoV-2 spike S1 protein induces potent virus-neutralizing antibodies at much higher levels than seen in the sera of convalescent patients.
Society
Brown RC, Kelly D, Wilkinson D, Savulescu J. The scientific and ethical feasibility of immunity passports. Lancet Infect Dis 2020, published 16 October. Full-text: https://doi.org/10.1016/S1473-3099(20)30766-0
Immunity passports are much less en vogue than during the first months of the pandemic: unethical and impractical, based on insufficient knowledge about what COVID-19 immunity, possibly a ‘perverse’ incentive and with doubtful economic benefits, and possibly discriminatory effects. If you want to read a pro-passport pleading about ‘people’s freedom’, check this paper. Not necessarily convincing. Maybe not a must-read.
Society, Prevention
The Editorial Board. End Our National Crisis. The New York Times 2020, published 16 October. Full-text: https://www.nytimes.com/interactive/2020/10/16/opinion/donald-trump-worst-president.html
The greatest threat to American democracy since World War II.
Spanish
If you read Spanish, read Valdés I. “No ser intubado cuando es imprescindible mata en minutos” – El País 2020, published 17 October. Full-text: https://elpais.com/espana/madrid/2020-10-16/no-ser-intubado-cuando-es-imprescindible-mata-en-minutos.html
Borja Quintana y Antonio Planas, presidente de la Sociedad Madrileña de Anestesiología, Reanimación y Terapéutica del Dolor y secretario de la Sociedad Española de este área, repasan la situación de su especialidad durante la pandemia.
French
If you read French, read Covid-19 : « L’hôpital n’a pas les moyens d’affronter la deuxième vague épidémique » – Le Monde 2020, published 17 October. Full-text : https://www.lemonde.fr/idees/article/2020/10/17/covid-19-l-hopital-n-a-pas-les-moyens-d-affronter-la-deuxieme-vague-epidemique_6056367_3232.html
Faute de moyens, la seule solution pour freiner la progression du virus repose sur le respect par tous des mesures barrières, selon la Fédération des infirmiers de réanimation. Un extrait : « Si les prévisions épidémiologiques se confirment, cette nouvelle vague sera plus haute et plus durable. Nous ne pourrons pas lui faire face, d’abord par manque de places en réanimation : le pays disposait de 5 000 lits de réanimation avant la première vague épidémique et c’est toujours le cas aujourd’hui. L’Etat annonce 12 000 lits de réanimation mobilisables, en ouvrant 7 000 lits supplémentaires… Mais ils ne sont pas équipés du matériel adapté pour ces soins complexes et aucune réserve de professionnels n’existe pour les ouvrir. »